Introduction
Urolithiasis has become a chronic disease with a significant impact on the quality of life and work situation of those who suffer from it. Its prevalence and recurrence rates are increasingly high, generating a significant socioeconomic impact in any country by affecting the health care system.
The lifetime risk of urinary stone (US) formation is around 10-15% in developed countries, but can reach 25% in the Indian subcontinent, the Middle East, and in parts of South America and Africa, with a recurrence rate close to 50%1–3. In Mexico, in 1984, the Mexican Social Security Institute4 reported a urolithiasis prevalence of 2.4 cases/10,000 inhabitants, mainly in the states of Yucatán, Puebla, and Quintana Roo, with 5.8 cases/10,000 inhabitants.
The formation and development of crystals in urine depend on the level of supersaturation and the amount of metabolic promoters and inhibitors. Numerous factors inherent to human biology and some sociodemographic variables have been identified that favor the development of US5,6.
The analysis of US has been established to identify their composition and prevent their formation, using various techniques ranging from the simplest, such as radiological analysis, to the most advanced and modern, such as crystallography, which is responsible for the qualitative and quantitative identification of US3.
Crystallographic analysis using the inverse Fourier technique is a technique that uses the Fourier transform to analyze the solid structure of crystals, which helps to understand their properties7. The Fourier transform is a mathematical tool used to represent the distribution of atoms in a crystal. To apply the Fourier transform in crystallography, it is performed using infrared spectroscopy, which is the most common technique in the study of the structure of solid materials. Through this analysis, precise information is obtained about the cause of urolithiasis by identifying and quantifying the crystalline phases using physical analysis methods to collect US from different urological treatments8.
In different parts of the world, there is research related to the analysis of US. For example, Daudon et al. in 20099 in France described that the crystalline organization is inversely proportional to the resistance to fragmentation by shock waves. Giannossi et al. in 201210 in Italy determined the mineralogical and morphological compositions of US, where calcium oxalate stones were the most frequent. In Belgium, Castiglione et al. in 201811 identified that the components of US were calcium oxalate monohydrate (COM) and dihydrate, anhydrous and dihydrate uric acid (UA), apatite, struvite, brushite, and other components. In Monterrey, Mexico, in 2013, Aragon-Tovar et al. in 201312 determined that the prevalence of US increases in summer, and that 80% of US had a mixed composition, with calcium oxalate plus calcium phosphate being the most frequent.
Thus, it is not surprising that different clinical guidelines on urolithiasis, published in recent years, do not hesitate to point out stone analysis as an essential element and starting point in the study of the disease13,14.
Therefore, the exclusive elimination of the US, without an adequate investigation of the causes that led to its formation, only means suppressing the expression of a disease that often causes new episodes. Thus, crystallographic analysis of the US is a useful tool to understand the causal mechanisms, as well as the therapeutic approach to lithiasis.
The objective of this study is to describe, through crystallography with the inverse Fourier technique, the chemical composition of US from adult patients treated with lithotripsy at the Centro Médico ABC in Mexico City.
Material and methods
With authorization from the local ethics committee of the American British Cowdray Medical Center in Mexico City under No. CMABC-24-52, we conducted a descriptive, observational, cross-sectional, and retrospective case series of records from 2013 to 2023, including adult patients of any gender, older than 18 years, who underwent lithotripsy and had their US analyzed by crystallography with the Fourier transform technique at the Mayo Clinic in the United States of America. Records of patients with chronic urolithiasis and known calcium and urea alterations were excluded. Those with incomplete measurements, impossibility of analyzing the US, or loss of the record were eliminated.
The size of the sample was by convenience, totaling 314 records.
For statistical analysis, mean and standard deviation, as well as frequency and percentages, were used.
Results
From 2013 to 2023, a total of 314 patients underwent lithotripsy, of whom 217 patients (69%) were men with a mean age of 46 ± 14 years; the rest were women with a mean age of 44 ± 16 years, resulting in a male-to-female ratio of 2.2-1.
Regarding the age group, the most frequent was 40-59 years with 43%, followed by 18-39 with 39.5%, then 60-79 with 14.3%, and the rest older than 80 years (Table 1).
Table 1. Gender distribution by age group
| Age groups (years) | |||||
|---|---|---|---|---|---|
| Variables | f (%) | ||||
| < 20 | 20 a 39 | 40 a 59 | 60 a 79 | > 80 | |
| Female | 5 (1.6) | 36 (11.5) | 41 (13) | 13 (4.1) | 2 (0.6) |
| Male | 0 | 88 (28) | 95 (30) | 32 (10.2) | 2 (0.6) |
| Total | 5 (1.6) | 124 (39.5) | 136 (43) | 45 (14.3) | 4 (1.2) |
f: frequency.
In relation to the months of presentation of urolithiasis (US), a homogeneous distribution was maintained from March to October, with a slight increase in March and July (Fig. 1).

Figure 1. Distribution by month and gender.
Regarding the crystallographic analysis, 60% (188 stones) had a unique composition. Of these, 46% were composed of COM; 11% by calcium oxalate dihydrate (COD), 2.7% by brushite (BS), and 2.1% by UA (Fig. 2).
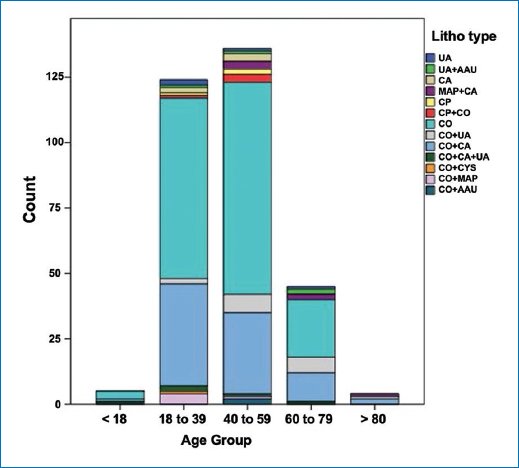
Figure 2. Distribution by type of composition and age group.
Calcium oxalate (CO) was the basis of the mixed composition of the stones, of which 27% were composed of a base plus carbonate apatite (CO + CA), 5.5% of a base plus UA (CO + UA), 5.5% of a base plus magnesium ammonium phosphate (CO + MAP), 1.3% of a base plus calcium phosphate (CO + CP), 2% of a base plus carbonate apatite plus UA (CO + CA + UA), 0.9% was formed of a base plus ammonium acid urate (CO + AAU), and the rest of a base plus cystine (CO + CYS) (Table 2).
Table 2. Distribution of single and mixed stone components by gender
| Type of composition | Male (%) | Female (%) | Total (%) |
|---|---|---|---|
| Single composition | |||
| COM | 108 (34.4) | 36 (11.6) | 144 (46) |
| COD | 31 (9.9) | 4 (1.1) | 35 (11) |
| Bru | 3 (1.05) | 2 (0.65) | 5 (1.7) |
| UA | 4 (1.1) | 0 | 4 (1.1) |
| Mixed composition | |||
| CO + CA | 56 (13) | 29 (9) | 85 (27) |
| CO + UA | 14 (4.4) | 3 (1.1) | 17 (5.5) |
| CO + MAP | 6 (1.9) | 1 (0.4) | 7 (2.3) |
| CO + CA + UA | 2 (0.65) | 4 (1.3) | 6 (2) |
| CO + CP | 2 (0.65) | 2 (0.65) | 4 (1.3) |
| CO + AAU | 0 | 3 (0.95) | 3 (0.95) |
| CO + CYS | 3 (0.95) | 0 | (0.95) |
COM: calcium oxalate monohydrate, COD: calcium oxalate dihydrate, Bru: brushite; UA: uric acid, CO + CA: calcium oxalate + apatite, CO + UA: calcium oxalate + uric acid; CO + MAP: calcium oxalate + ammonium magnesium phosphate (struvite), CO + CA + UA: calcium oxalate + apatite + uric acid; CO + CP: calcium oxalate + calcium phosphate; CO + AAU: calcium oxalate + ammonium acid urate; CO + CYS: calcium oxalate + cysteine.
Discussion
Urolithiasis is a common disease that represents a healthcare burden with associated risk factors. This study collected information from patients treated for urolithiasis for the 1st time, with a male/female ratio of 2-1, which is consistent with what Awedew et al. reported15 worldwide and Aragon-Tovar in Mexico12.
The age range with the highest frequency was 40-59 years, with a mean age of 46 ± 14 years. This result is consistent with Arias16 and Awedew15 who report that urolithiasis develops most frequently between the fourth and sixth decades of life.
Regarding the month of presentation of lithiasis, it occurred homogeneously from March to October, which does not correspond to that reported by the Instituto Mexicano del Seguro Social17, by Aragon12 who states that the incidence rate of US increased in the hottest months.
An adequate analysis of the US through crystallography is essential to initiate the diagnostic process of the underlying metabolic alteration. Reports on the composition of the stones mostly indicate that > 70% have a mixed composition12,17, but in our study, only 40% were of mixed origin, with those of unique composition predominating.
In stones of unique composition, the highest percentage in men was COM, which is generally associated with idiopathic hypercalciuria18.
COD was more prevalent in the group of women, which is associated with a deficiency of crystallization inhibitors (phytates) and a greater presence of heterogeneous nucleants in the urine, which are organic matter induced by diseases more prevalent in this group, such as arterial hypertension and hyperuricemia, and to a lesser extent, hyperglycemia and hypercholesterolemia19.
UA stones followed, which are associated with a disorder of purine metabolism caused by a hereditary deficiency of the xanthine dehydrogenase/oxidase enzyme, leading to an increase in xanthines; finally, brushite stones are associated with primary hyperparathyroidism or primary tubular acidosis20.
It is known that mixed component stones are due to heterogeneous nucleation phenomena (urate nuclei) with a calcium oxalate base, which is produced daily in the liver or through diet. Calcium oxalate kidney stones form when urine contains more of these substances than the fluid can dilute them; when this occurs, calcium and oxalate form crystals, and if at that moment the urine lacks the substances that prevent the agglutination of crystals, then the ideal environment for the formation of kidney stones is created. The consumption of nuts, chocolate, Vitamin C, and dietary supplements has a high oxalate content. Furthermore, intestinal bypass surgery, excessive use of calcium-based antacids and laxatives, and certain drugs used to treat migraines or depression increase calcium oxalate levels20.
In this study, the base of the mixed component stones is calcium oxalate, with the most frequent combination, in both men and women, being calcium oxalate plus carbonate apatite (CO + CA); apatite is the type of crystal found in bones, formed from the union of ammonium and carbonate radicals with phosphate in the urine, and is more common in middle-aged men. It is related to hyperparathyroidism.
Following in frequency are calcium oxalate plus UA (CO + UA) stones, presenting more frequently in men. Next are calcium oxalate plus magnesium ammonium phosphate (CO + MAP) stones; magnesium ammonium phosphate (struvite) stones are often called “infection stones” because they are closely related to urinary tract infections caused by organisms that break down urea. They can grow rapidly over a period of weeks to months and, if not treated properly, can become staghorn or branched stones that fill the entire collecting system of the kidney. If left untreated, they can lead to impaired kidney function and end-stage renal disease21.
The most important aspect of the present study is to offer a description of the type of stone components that patients treated in a private tertiary-level hospital present, paving the way for prospective studies where, in addition to knowing the components of the stones, comorbidities, diet type, etc., are associated, since kidney stones are sometimes the initial clinical signs of other diseases.
Conclusion
The importance of crystallographic analysis is essential for the medical treatment of patients with urolithiasis because it allows the recognition of diseases associated with the components of the stone and therefore, their adequate treatment and prevention.
Funding
The authors declare that they have not received funding.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical considerations
Protection of humans and animals. The authors declare that no experiments involving humans or animals were conducted for this research.
Confidentiality, informed consent, and ethical approval. The authors have obtained approval from the Ethics Committee for the analysis of routinely obtained and anonymized clinical data, so informed consent was not necessary. Relevant guidelines were followed. Due to the type of study, clinical records were reviewed, which required authorization from the local Ethics Committee of the American British Cowdray Medical Center in Mexico City under No. CMABC-24-52.
Declaration on the use of artificial intelligence. The authors declare that no generative artificial intelligence was used in the writing of this manuscript.
