Introduction
Anesthetic treatment in neonates and microneonates can be complex, due to the anatomical and physiological characteristics of these patients, in addition to not having the necessary elements to carry out adequate monitoring.
This group of patients has greater morbidity and mortality during anesthesia, so it is important that the anesthesiologist knows the most important aspects to offer safe and effective anesthetic treatment1,2.
The neonatal period covers from birth to the first 28 days of life and a baby born before 37 complete weeks of gestation (WOG) is considered a microneonate3.
The American association of pediatrics4 defines age corrected for gestational age as the result of gestational age plus chronological age, so neonates are classified as:
- − Post-term from 41 to 43 WOG
- − Term from 37 to 40 WOG
- − Mild preterm from 32 to 36 WOG
- − Moderate preterm from 28 to 31 WOG
- − Severe preterm in children under 28 WOG.
Physiological characteristics in preterm and term newborns (NB)
Cardiovascular system
There are three prenatal shunts (ductus venosus, ductus arteriosus, and foramen ovale) that are necessary for the preferential distribution of blood flows. After birth, NBs present a transitional circulation and pressure changes that allow the physiological and anatomical closure of the shunts4.
- − Foramen ovale closes functionally a few minutes after birth due to reduced venous return and subsequent decreased pressure in the right atrium. There is also an increase in pulmonary flow and an increase in pressure in the left atrium. Thus, the higher pressure of the left atrium compared to the right atrium, together with the negative intrathoracic pressure, functionally closes the foramen ovale. Anatomical closure occurs over the next 3 months
- − Venous duct is functionally closed during clamping of the umbilical cord by the action of fibrin. Anatomical closure occurs 7-14 days after birth, in preterm NB (PTNB) closure is slower
- − Ductus arteriosus closes functionally at 6 h of life due to the decrease in prostaglandins and the increase in arterial O2 and anatomically closes at 6 weeks
- − Patent ductus arteriosus is more common in PTNB weighing < 800 g
- − During anesthesia these shunts can reopen due to hypothermia, hypercarbia, acidosis, inadequate fluid management, or respiratory distress syndrome (RDS), which increases pulmonary arterial pressure causing congestive heart failure and necrotizing enterocolitis (NEC)5
- − Cardiac output (CO) is defined as heart rate (HR) multiplied by systolic volume; in neonates output depends on HR; thus, it should besot maintained between 120 to due to 180 beats/min (bpm). CO decreases during bradycardia and tachycardia due to decreased diastolic filling and stroke volume3
- − The parasympathetic innervation of the heart is highly developed in neonates, and therefore, risk of bradycardia increases
- − Systemic blood pressure varies with post-gestational age of the newborn and normalizes at 36 h. Arterial hypotension develops faster lower the patient’s weight mainly due to immaturity of the autonomic nervous system6.
- To know should the appropriate values for mean arterial pressure (MAP), the following formula must be used2:
- MAP = gestational age + 5 mmHg
Respiratory system
- − Pulmonary vascular resistance (PVR) decreases at birth, because alveoli fill with air resulting in compression of the alveolar capillaries, which, in turn, favors the flow of pulmonary circulation4
- − When hypoxia occurs, PVR increases, causing a right-to-left shunt7, systemic venous blood does not pass through the pulmonary circulation, and therefore, low oxygen blend mixes with oxygenated blood and ultimately reaches the left heart from the pulmonary veins, this mixed, hypoxemic blood reaches the aorta and the systemic arterial circulation, resulting in cyanosis of the skin and mucous membranes7,8
- − The thorax is a structure made of cartilage and the muscles which make breathiest possibility are not fully developed, making it result in on increased risk of volume loss in the lungs. This condition favors closure of the airway, especially if they apnea, to sedation or on neuromuscular blockade8,9
- − The lung is less distensible, which facilitates the physiological closure of some less ventilated areas and, in turn, a greater tendency to form atelectasis, due to bronchial tree has fewer branches that make up10
- − Respiratory mechanics depend mainly on the diaphragm, with only 10% of type I fibers in PTNB compared to 25% in those NB who reached term4. This discrepancy results in a decreased residual capacity and causes respiratory depression11,12
- − Surfactant factor (SF) is released into the alveolus to maintain alveolar compliance and functional residual capacity (FRC) during expiratory phase. Before 32 to 34 WOG, there is little SF production which predisposes to RDS in the preterm newborn. Minute ventilation is higher in the neonate so induction and awakening with halogenated agents is faster. Premature patients have high metabolic activity, with oxygen requirements of 6-8 mL/kg/min compensating with high respiratory rates (RF), which is why they do not tolerate apneas for more than 30-45 seconds11,13
- Microneonates may require mechanical ventilation and oxygen supplementation for prolonged periods of more than 28 days, so they may develop bronchopulmonary dysplasia (BPD)5.
- − Apneas in premature babies and neonates. Apneas can be of central origin, obstructive or mixed. The most common causes of perioperative apnea are hypoglycemia, hypoxia, anemia, hypothermia, electrolyte alterations, BPD, NEC, or sepsis
This group presents periodic breathing with pauses of no more than 20 s. When the respiratory pause is longer and is accompanied by bradycardia and/or cyanosis, paleness, and hypotonia, it is termed pathological apnea3,5.
Pathological apnea occurs in up to 45% at 48 WOG post-conception, and decreases by up to 1% after 56 WOG; thus, these patients should not be managed as outpatients before 60 WOG2,3.
Renal system
In preterm patients with the same gestational age, renal function may differ due to pathology or differences in postnatal age. Birth weight does not influence the assessment of glomerular filtration (children with the same weight but different gestational age will have different kidney function).
From the 35th week of gestation, the glomerular filtration rate is 35% and responds little to aldosterone, resulting in difficulty in reabsorbing sodium and bicarbonate, thus, increasing the risk of hyponatremia and metabolic acidosis. By the 3rd or 4th day of life, the glomerular filtration rate increases due to high CO and reduced resistance in the blood vessels of the kidneys. The kidneys, under these conditions, can still respond to changes in CO by concentrating or diluting urine; however, their capacity is limited, since if the fluid intake is reduced, the risk of rapid dehydration of the baby increases4,5,10.
Liver system
Hepatic clearance through P450 isoenzymes reaches 85% maturity at 44 weeks of age. Premature babies have lower liver glycogen reserves, which develop in the last WOG. Gluconeogenesis pathways are underdeveloped, producing a greater risk of hypoglycemia. Reduced binding to albumin and α-acid glycoprotein results in a higher concentration of free drug, prolonging the effect of medications. It can also be prolonged due to the immaturity of the enzyme systems, inadequate renal excretion, and well as hypothermia5.
Central nervous system
In infants with open fontanelles, cerebral perfusion pressure varies according to blood pressure. Autoregulation occurs when the MAP decreases by 20% of the initial value4. Altered vascular autoregulation due to hypoxia, perinatal asphyxia, pneumothorax, RDS, mechanical ventilation, and arterial hypotension increases the risk of intraventricular hemorrhage, which occurs up to a 50% of infants have low birth weight and leads to the formation of periventricular leukomalacia5.
Hematopoietic system
The full-term newborn has a hemoglobin of 18-20 g/dL, as compared to 13-15 g/dL, in the preterm baby in which 70-80% is in the form of fetal hemoglobin (HbF).
The increased blood volume (BV), CO, and elevated HbF concentration compensates and maintaining a HCT of 40-45% is essential3,5.
Thermoregulation
Full-term and pre-term NB have decreased brown fat reserves, and their skin does not have keratin. If we add to this the high ratio between body surface area and body weight, this age group tends lose heat more quickly.
Temperature measurements should be adequately monitored therefore5.
Hypothermia can cause hypoglycemia, apneas, and metabolic acidosis and, in addition to delaying drug metabolism, can cause cardiovascular stress, which in itself is an indirect indicator of excessive insensible water loss3,14.
It is important to avoid heat loss during transfer from the neonatal intensive care unit to the operating room during a surgical anesthetic event3.
Techniques to maintain body temperature should include waterproof bandaging with padding and/or cotton on exposed areas, placement of radiant heaters and/or forced air warming units, warm intravenous fluids should be warm, as well warming and humidifying inspired gases15.
Pain
In 1987, Anand et al.16 demonstrated that PTNB not only feel pain but also integrate with somatic, neuroendocrine, and autonomic areas of the brain during gestation. All the aforementioned can results in an exaggerated response to pain and stress.
Perioperative management
Preanesthetic assessment
The objective during the pre-operative assessment is to collect information about gestational age, corrected age and weight, emphasizing perinatal history such as complications during pregnancy, a score that records the physical condition of a baby just after it is born, the presence of perinatal asphyxia, heart disease detected up to that moment, sepsis, meconium aspiration, periods of hypoglycemia or seizures, requirement for supplemental oxygen, and/or mechanical ventilation requirements, including fraction of inspired oxygen (FiO2) and ventilatory parameters.
- − Physical examination: there are no assessment scales like those developed for adults; however, micrognathia or facial dysmorphisms could indicate difficulty with ventilation or intubation8. Search for signs of respiratory distress, such as nasal flaring, sternal, subcostal, intercostal retraction, and tachypnea, observes capillary refill, color, turgor of the skin and mucous membranes, and systemic blood pressure, which are indicators of the patient’s hydration and perfusion status
- − In the case of term and PTNB, vascular access can be peripheral or central. The choice of the type of vascular access depends on the patient’s needs:
- − If the neonate needs vascular access at birth, an umbilical catheter can be used
- − If the neonate is stable and needs vascular access, a short catheter, an epicutaneous cava or peripherally inserted central catheter (PICC), or a central access can be used
- − If the neonate is not stable, central access, a centrally inserted central catheter of high flow or femoral PICC should be used
- − It is important that the vascular accesses are correctly fixed since it is easy to lose them during surgeries or during transfer to the operating room2,4,8
- − All premature NB should undergo an echocardiogram before the surgical event, emphasizing malformations related to prematurity, since sometimes their sequelae can directly impact anesthetic management5
- − Fasting guidelines suggest 4 h for formula, 3 h for breast milk, and 1 h for clear liquids17. Exceeding this time in NB does not increase the safety profile against bronchoaspiration, but it does increase irritability, dehydration, and hypotension that is difficult to control during induction along with hypoglycemia, increasing the production of ketone bodies with a decrease in bicarbonate causing acidosis metabolic
- − The entire surgical team must know the surgical and anesthetic plan, the probable duration of the procedure and the risk of blood loss greater than usual, which is why it is essential to have good communication between all members and thus reduce complications.
Operating room
- − The operating suite must have a temperature of 24-27°C to avoid hypothermia in the newborn. In the microneonate patient, ideally, temperature should therefore remain constant, and surgery should by performed in a radiant crib18
- − Monitoring: always measure HR, RF, peripheral oxygen saturation SPO2, ideally with two oximeters, one in the right hand and one in the left arm or in a pelvic limb (to assess pre- and post-ductal oxygenation), end-expiratory carbon dioxide (ETCO2), non-invasive arterial pressure with a cuff suitable for the size of the patient, and thermometer
- − Invasive monitoring: it depends on the type of surgery, especially in surgeries with high volume turnover (> 40 mL/kg), with a central catheter and an arterial line to be able to follow hemodynamic changes, acid base, and hemoglobin levels in real time19,20
- − Airway preparation: NB are more susceptible to airway-related adverse events, so alternative strategies are required for successful and safe intubation3,12,18
- − Ventilation is crucial to manage the airway in any person. The face mask should be properly sized to avoid leaks, properly covering the mouth and nose without injuring the eyes
It is important to elevate the shoulders using any support mechanics and keep the head in a neutral or slightly tilted back position, avoiding excessive tilt that could block the airway. It is also important not to apply inspiratory pressures above 15-16 cmH2O during manual ventilation to prevent air from entering the stomach. If necessary, size 0 and 00 orotracheal cannule can be used8,21 (Fig. 1).
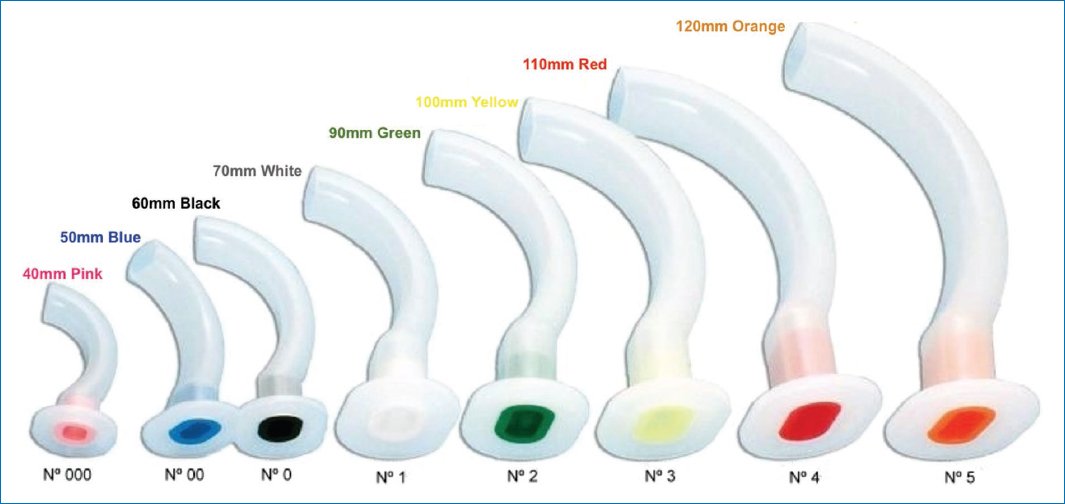
Figure 1. Sizes of Guedel cannulae.
- − Maintain a deep anesthetic plane with adequate analgesia and muscle relaxation, thereby decreasing the risk of laryngospasm and improving the field of vision for intubation on the first attempt8,22
- − Avoid prolonged periods of hypoxemia because they trigger bradycardia and cardiac arrest. It has been shown that oxygen administration while performing laryngoscopy preserves normal oxemia longer than the conventional technique12
- − Studies suggest that the best way to intubate the airway is to use video laryngoscopes, with this device an 80% success rate is obtained on the first attempt in children under 10 kg and in NB with a difficult airway8,15
If endotracheal intubation is being performed by direct laryngoscopy, Miller 00 blades can be used in the microneonate, while Miller blade 0 is adequate for premature NB and Miller blade #1 works for NB (Fig. 2).
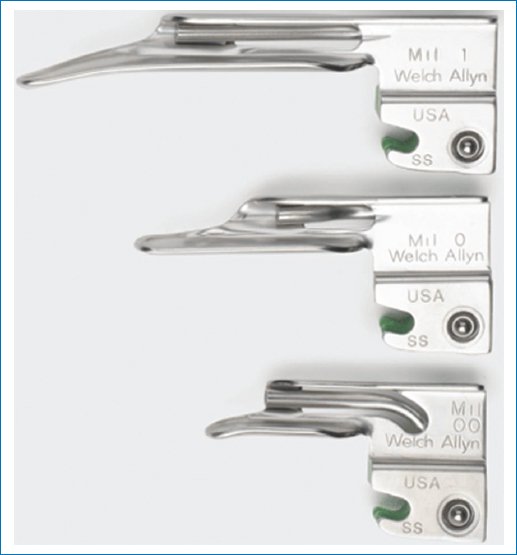
Figure 2. Miller-type laryngoscope blades.
Supraglottic devices are helpful in case of difficult ventilations or in procedures that do not require prolonged ventilatory support8.
The choice of endotracheal tube is determined by the weight of the NB as well as the clinical judgment of the anesthesiologist, choose the largest possible to decreased airway ressitance3,8 (Table 1).
Table 1. Endotracheal tube size and fixation length at labial commissure based on weight
| Weight (kg) | Oral length (cm) | Tube size (mm) |
|---|---|---|
| < 0.7 | 5 | 2 |
| < 1 | 6 | 2.5 |
| 1 | 7 | 2.5-3 |
| 2 | 8 | 3 |
| 3 | 9 | 3-3.5 |
| 3.5 | 9.5-10 | 3-3.5 |
Balloon tubes are not contraindicated. The benefits of their use are the reduction of ventilation leaks, the need for frequent airway manipulation, and the reduced risk of aspiration3,8,23.
To calculate the distance of the tube from the labial commissure, use the following formula weight (kg) + 69.
The mechanical ventilation mode that best suits the patient should be chosen, with a tidal volume of 5-7 L/min, with positive end-expiratory pressure of 5-7 cmH2O, with maximum inspiratory pressure of 25 cmH2O, using the lowest amount of FiO2 that the patient can tolerate to maintain an SPO2 between 88% and 95%, and a RR of 30-50 rpm8–10.
Fluid requirements in this period are low, but insensible evaporative losses should be prevented, the BV of the term NB is 80 mL/kg and in preterm of 100 mL/kg. For fluid replacement, “balanced solutions” such as lactated Ringer’s lactate and plasma-Lyte, which contain metabolic anion precursors of bicarbonate, are preferred14,20,24,25.
In PTNB patients, acetate is preferred to lactate due to its rapid metabolism and lower oxygen consumption26.
To avoid hydric overload, it is suggested to use 5% albumin from the beginning, with a maximum dose of 1 g × kg for the entire surgical event25.
- − The NB and PTNB with normotension and hypoperfusion should be treated with crystalloid and colloid administration in addition to inotropic and vasopressor drugs14
- − If all of the above is inefficient, blood transfusion is necessary, as well as the use of antifibrinolytics20–30
- − Approximately 4 ml/kg of the globular pack increases the patient’s hemoglobin by 1 g/dL25. In general terms, achieving a hemoglobin of 13-14 g/dL is a useful goal while avoiding a very high hematocrit (> 65%) because increasing viscosity reduces tissue oxygen delivery and is associated with complications12
- Fresh frozen plasma is transfused at 12-15 mL/kg and platelets at 10-20 mL/kg; however, its use is restricted to NBs with diagnosed coagulation disorders14,20.
Neonatal massive transfusion is defined as transfusion of a circulating volume in 24 h or 50% of the circulating volume in < 3 h30.
Another way to calculate the volume of packed red blood cells to transfuse for NB is:
Volume to transfuse (mL) = Hb (g/dL) required × weight (kg) × 414,31
Finally, to avoid accidental fluid overload, pressure-limited infusion pumps with syringes up to 50 mL should be used in the neonate, and proper flushing of lines and infusers should be monitored to prevent air embolism20.
Conclusion
Anesthetic management of NB and microneonates depends on a clear understanding of the limitation of each group, considering age, health status, and type surgery that each will undergo.
Thus, it is critical to understand the effects of all anesthetics used, possible complications and the necessary treatments to be employed.
The well-trained anesthesiologist provides adequate preparation and planning of anesthesia for NB and thus reduces complications before, during and after neonatal surgery.
Funding
The authors declare that they have not received funding.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical considerations
Protection of humans and animals. The authors declare that no experiments involving humans or animals were conducted for this research.
Confidentiality, informed consent, and ethical approval. The study does not involve patient personal data nor requires ethical approval. The SAGER guidelines do not apply.
Declaration on the use of artificial intelligence. The authors declare that no generative artificial intelligence was used in the writing of this manuscript.
