Introduction
Ischemic heart disease is the main cause of preventable death worldwide, and in Mexico it is the second cause of mortality, clinically it has different expressions, one of them is stable angina1–4. The term ischemic heart disease is used to represent the presence of inadequate blood supply to the myocardium due to obstruction of the epicardial coronary arteries. Chronic coronary syndrome (CCS), also known as stable ischemic heart disease (ICD), is based on a classic history of angina pectoris in the presence of cardiovascular risk factors or known coronary artery disease5,6, the most common pathophysiological mechanism is the atherosclerotic obstruction of one or more epicardial coronary arteries7–10.
Insufficient blood supply to the myocardium produces angina, dyspnea, myocardial infarction, arrhythmia, left ventricular dysfunction, shock, and death11,12.
The diagnosis of CCS is confirmed by non-invasive stress tests or by invasive evaluation of the coronary circulation, which determines the severity of the disease to also establish the prognosis and guide treatment: medical therapy, percutaneous revascularization (PCI) or surgical revascularization (CABG)13–15. Non-invasive methods for the evaluation of stable angina include single photon emission computed tomography (SPECT)16,17 and echocardiography18–21. Even though there is evidence that directly correlates obstructive CAD with functional alteration measured by echocardiography22, these alterations have not been correlated with the degree of myocardial ischemia evaluated with non-invasive methods using the SPECT technique.
Nuclear cardiac imaging is the reference technique for the non-invasive evaluation of myocardial perfusion, cardiac SPECT has become the most frequently performed imaging technique for the functional evaluation of patients with ischemic heart disease, having an association with the presentation of adverse cardiac events17,23. Strain echocardiographic imaging can quantify regional and global myocardial function, specifically quantifying multiplanar left ventricular (LV) function, including longitudinal, radial, and circumferential contraction24–27. Two echocardiographic modalities have been used to quantify strain, based on tissue Doppler (TDI) and 2D speckle tracking strain28, normal levels depend on the equipment and software of each provider29. Deformation imaging has the advantage of differentiating active contraction from passive motion, ischemic wall motion abnormalities are often associated with passive motion, such as passive expansion, recoil, and clamping of adjacent segments30–32.Global longitudinal strain (GFD) has been correlated with LV infarction size in patients with acute myocardial infarction, it is a predictor of LV remodeling and adverse events (heart failure and death)22,33–38.
This study attempts to demonstrate that patients with stable angina and myocardial perfusion abnormalities on cardiac SPECT will also have abnormalities in global longitudinal strain, since global longitudinal strain can be affected prematurely by repeated episodes of ischemia.
Methodology
The study was approved by the ethics committee of the ABC Medical Center, subject to the disposals of the Declaration of Helsinki of 1964 and the disposals of the General Health Law on research, protecting the confidentiality of the information through an informed consent form.
All the authors participated in the design of the study and had access to the data, which were analyzed by the clinical research area of the ABC Medical Center, guaranteeing the accuracy and integrity of the data analysis and the fidelity of the study. The manuscript was written by the first and second authors and reviewed by all authors.
This is a retrospective, descriptive, observational case-control and cross-sectional study from march 2018 to may 2021 at the ABC Medical Center, Observatory Campus.
Patients with Stable Angina (SA) at the ABC Medical Center undergoing a myocardial perfusion study with the SPECT technique, who have a study of Global Longitudinal Strain measured by Echocardiography. Within the group of cases, patients with SA from the ABC Medical Center who underwent a cardiac SPECT positive for ischemia, who have a study of Global Longitudinal Strain measured by Echocardiography, and the control group, patients with SA of the ABC Medical Center who underwent a cardiac SPECT negative for ischemia, who have a study of Global Longitudinal Deformation measured by Echocardiography.
Inclusion criteria: patients with stable angina with at least 2 months evolution, with angina, over 18 years of age, undergoing a myocardial perfusion study with SPECT technique in the rest and stress phase (physical or pharmacological) and measurement of Global Longitudinal Strain by echocardiography. The exclusion criteria.
Exclusion criteria: Patients with a history of myocardial infarction, primary cardiomyopathies (hypertrophic, restrictive, dilated, non-compaction, infiltrative), moderate/severe valve disease, atrial fibrillation during echocardiography and/or SPECT, chronic kidney disease (CKD), history of cardiac surgery, history of cancer and/or those who had received chemotherapy.
The clinical variables were collected from the electronic file and from the database of the nuclear cardiology service of the ABC Medical Center, according to the classification of Diamond et al. In 1979, three criteria for chest pain were considered:
- Retrosternal chest pain.
- Pain that occurs with physical activity.
- Pain that disappears when resting or taking nitrates.
Based on the above, chest pain was classified into three types:
- Typical angina: Presents 3 criteria.
- Atypical Angina: Presents 2 criteria.
- Non-anginal chest pain: Presents 1 criterion.
Stable myocardial ischemic disease will be defined as those patients with a history of chest pain (typical and atypical angina and non-anginal chest pain) and/or at least two months evolution dyspnea who underwent myocardial perfusion SPECT and presented perfusion defects in the stress phase compatible with ischemic heart disease.
Subsequently, the pretest probability was calculated according to the 2019 European Heart Association (ESC) Chronic Coronary Syndrome guideline.
Data were collected directly from the electronic file and from the database of the nuclear cardiology service of the ABC Medical Center of patients with stable angina who underwent myocardial perfusion SPECT in the rest and stress phase; and the Xcelera system in a period from 2018 to 2021.
The time of evolution of angina, comorbidities, pretest probability and cardiac SPECT results, such as LVEF and walls with perfusion defects, in patients with stable angina, were captured in an electronic spreadsheet, in a period from 2018 to 2021.
To perform the echocardiogram, the patients attended scheduled the cardiovascular center of the ABC medical center. A transthoracic echocardiogram was performed on suspicion of stable ischemic heart disease, with Philips CX-50, iE 33, and EPIQ 7 equipment. The acquisition of the images was carried out by different cardiologists specialized in echocardiography, who carried out the structural and functional analysis according to the ASE guidelines, calculating the global longitudinal deformation by the speckle tracking method and for the purposes of the study the regional longitudinal strain was calculated to each of the myocardial walls based on the 17-segment polar map.
To perform the cardiac SPECT, the patients attended scheduled the nuclear medicine service of the ABC medical center. Myocardial perfusion studies with the SPECT technique were performed using Technetium 99m as radiotracer. The protocol was carried out in a single day. The stress phase was performed with physical effort on a treadmill, or with dipyridamole according to the clinical characteristics of the patient.
Stress images were acquired with the SPECT technique synchronized to the electrocardiogram (Gated SPECT).
The primary outcome was to assess whether there is a correlation between Global Longitudinal Deformation and myocardial perfusion SPECT in patients with CCS. The secondary outcome was to establish whether there is a regional correlation between the myocardial segments with ischemia and low Global Longitudinal Deformation.
The nominal and dichotomous variables were reported in frequencies and percentages, the quantitative variables with normal distribution with means and standard deviation, those with non-parametric evaluation through median and minimum and maximum values. The analysis of global prevalence, of alterations in the measures proposed by Strain and the prevalence ratio (PR) of the presence of ventricular dysfunction was calculated, which indicated how many times more likely it is that individuals with low GFD have a defect in perfusion in myocardial SPECT compared to those who did not present it. If the ratio was greater than 1, it indicated the times that it is more likely to present some alteration by means of the Strain analysis when studying patients with stable angina. The prevalence odds (OP) were analyzed, and the prevalence odds ratio was calculated. The comparative analysis of dichotomous and nominal variables was performed using the X2 test or Fisher’s exact test (the latter if the observed box is less than 5). In the case of comparison of means, between two groups they were made according to their normality distribution (Shapiro Wilkins or Kolmogorov Smirnoff) and the Student’s T or Mann Whitney U test was performed. In the comparison of more than two groups, ANOVA or Kruskall Wallis. For predictive analysis of binary logistic regression. Statistical significance when p < 0.05 Excel and SPSS 19 software were used.
Results
In these patients, 40 met the inclusion criteria, the rest were excluded, the patients were divided into cases (n = 21) and controls (n = 19). 21 men and 19 women were evaluated, the mean age was 70 years (range 38-89 years), the mean body mass index (BMI) was 26.5 (range 20-46), 58% of the patients had a family history of ischemic heart disease and most (53%) did not perform regular physical activity (57% cases vs 47% controls). The demographic characteristics of the patients are shown in Table 1. Among the comorbidities, systemic arterial hypertension stands out in 60% of the subjects evaluated and hypercholesterolemia in 55%; 70% of the participants denied smoking. All patients underwent cardiac SPECT at rest, the stress phase was performed with pharmacological stress in 47% of the participants and physical stress in a treadmill in 53%. The study was performed in order to the following symptoms: Chest pain in 83% and dyspnea in 18% of the participants. According to the characteristics of chest pain, 33% of the participants referred it as typical angina, 20% as atypical angina, and 33% as non-anginal chest pain. All patients presented LVEF > 50% and normal mobility in all myocardial walls.
Table 1. Characteristics of patients at baseline
| Total (n = 40) | Controls (n = 19) | Cases (n = 21) | p | |
|---|---|---|---|---|
| Male sex | 21 | 7 | 14 | 0.059 |
| Female sex | 19 | 12 | 7 | |
| Age | 70 (38-89) | 73 (43-89) | 64 (38-80) | 0.01 |
| Weight | 75 (44-118) | 69 (44-110) | 77 (58-118) | 0.03 |
| Height m | 1.68 (1.5-1.81) | 1.64 (1.501-80) | 1.70 (58-118) | 0.007 |
| Body mass index | 26.5 (20-46) | 26.3 (20-36) | 27.3 (21-46) | NS |
| Family history | 23 (58) | 11 (57) | 12 (57) | NS |
| Physical activity | ||||
| No physical activity | 21 (53) | 9 (47) | 12 (57) | NS |
| Aerobic exercise | 19 (47) | 10 (53) | 9 (43) | NS |
| Comorbidities | ||||
| Non-smoking | 28 (70) | 15 (79) | 13 (62) | NS |
| Current smoking | 11 (28) | 4 (21) | 7 (33) | NS |
| Suspended smoking | 1 (3) | 0 | 1 (5) | NS |
| Diabetes Mellitus | 6 (15) | 0 | 6 (29) | 0.02 |
| Hypertension | 24 (60) | 13 (68) | 11 (53) | NS |
| Hypercholesterolemia | 22 (55) | 11 (57) | 11 (53) | NS |
| Hypertriglyceridemia | 12 (30) | 6 (32) | 6 (29) | NS |
| COPD | 1 (3) | 0 | 1 (5) | NS |
| Reason for requesting the imaging study | ||||
| Chest pain | 33 (83) | 14 (74) | 19 (90) | 0.02 |
| Dyspnoea | 7 (18) | 5 (26) | 2 (10) | NS |
| Clinical Characteristics of chest pain | ||||
| Typical angina | 13 (33) | 4 (21) | 9 (43) | 0.06 |
| Atypical angina | 8 (20) | 6 (32) | 2 (10) | NS |
| Chest pain | 13 (33) | 4 (21) | 9 (43) | 0.07 |
| Dyspnoea | 6 (15) | 5 (26) | 1 (5) | 0.08 |
In the group of cases (n = 21), 18 subjects (85.7%) presented perfusion defects compatible with mild ischemia, 10 subjects (47.6%) with moderate ischemia, and 13 subjects (61.90%) presented perfusion defects compatible with infarction with mild schemia on residual tissue. There were no subjects with severe ischemia or infarction with moderate or severe ischemia in the residual tissue.
According to the myocardial wall with perfusion defects, it was evident that the inferior wall was the most affected with mild ischemia in 6 subjects (29%), in the inferior wall 4 subjects presented moderate ischemia (19%) and 4 subjects (19%) mild ischemia, in the septal wall the majority of subjects presented moderate ischemia (19%), in the lateral wall 4 subjects presented mild ischemia (19%). Regarding the infarction with mild ischemia, it was presented in 14% in the apex, and anterior and septal walls.
Global longitudinal strain (GLS) was measured in all study participants and was normal in both groups, in cases the GLS was −21 (range −15 to −29) and in controls it was −21 (range − 15.2 to −26), this shows that for the primary outcome, global longitudinal deformation is not affected in patients with stable angina and mild and moderate ischemia assessed by cardiac SPECT (Table 2 and Fig. 1). Regional longitudinal strain (RLS) was assessed in each myocardial wall and correlated with SPECT data in the same region in both groups (cases and controls) (Fig. 2).
Table 2. Evaluation of the apex by spect and its relationship with measurement by echocardiography using segmental longitudinal strain (apex)
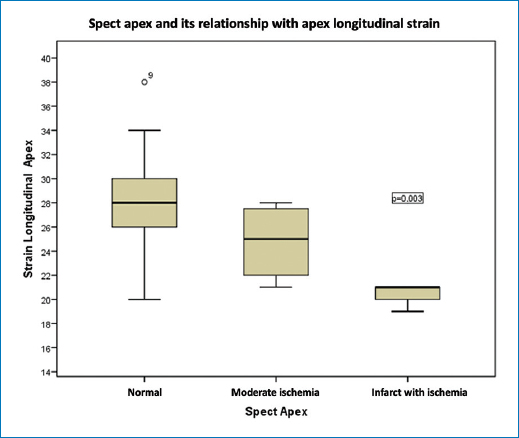
Figure 1. In the apex, the RLS was −27 ± 4 in controls and −29 (−20 to −34) in cases with normal SPECT; in patients with moderate ischemia, the RLS was −25 (Range −21 to −28) p 0.003 (Table 2).
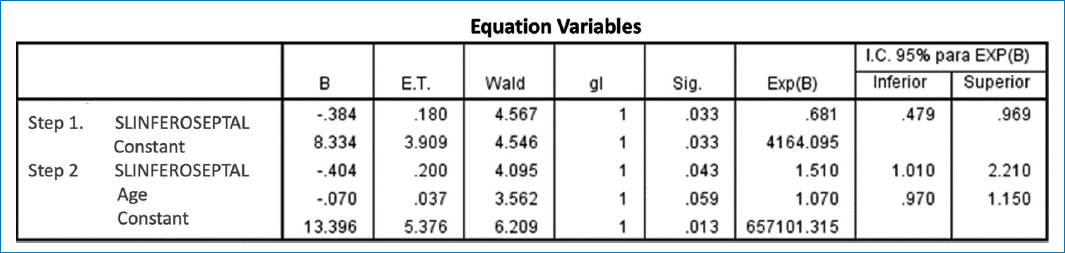
Figure 2. A binary logistic regression was conducted step by step, in which a model with significant variables was included. The results showed that the only important variable was the decrease in inferoseptal GLS, which was associated with a risk of 1.5 (95% confidence interval: 1.01-2.2) and statistical significance with p = 0.04.
In the Apex, the RLS was -27+/−4 in controls and –29 (range −20 to −34) in cases with normal SPECT; in subjects with moderate ischemia the RLS was −25 (range −21 to −28) p 0.003. In the anterior wall, the RLS was −21 (range −15.3 to −27) in controls vs 22.1 (range −16.1 to −27) in cases with normal SPECT. In subjects with mild ischemia the RLS was −22.1 (range −16 to −27), in moderate ischemia it was −20.8 (range −19 to −23.6) p 0.02. In the lateral wall, the RLS was −22.5 in controls vs −20.5 (range −18.6 to −26.5) in cases with normal SPECT. In subjects with light ischemia the RLS was −20 (range −18 to −22), in subjects with myocardial infarction with residual light ischemia the RLS was −15 p. 0.057. In the lower wall, the RLS was −21 in controls vs −22 (range −21 to −25.6) in cases with normal SPECT. In subjects with mild ischemia the RLS was −17.3 (range −16.8 to −19.1), in moderate ischemia it was −17.3 (range −17 to −17.6.) and in subjects with myocardial infarction with residual light ischemia the RLS was −13.8 (−8.1 to −19.6) p. 0.0001.
In a binary logistic regression model (Table 3), it was found that the LRS of the inferoseptal region is the one that best predicts ischemic heart disease and best correlates with cardiac SPECT (Correlation 0.52 p 0.01).
Table 3. Correlation between SPECT evaluated by segments and strain evaluated by segments: P correlation
| SL Apex | SPECT Apex | 0.50 | 0.001 |
| SL Anterior | SPECT Anterior | 0.39 | 0.01 |
| SL Anteroseptal | SPECT Anterior | 0.52 | 0.02 |
| SL Anterolateral | SPECT Anterior | 0.81 | 0.0001 |
| Sl Inferior | SPECT Inferior | 0.63 | 0.001 |
| SL Inferoseptal | SPECT Inferior | 0.52 | 0.01 |
| SL Anterolateral | SPECT Lateral | 0.51 | 0.001 |
Discussion
There is considerable interest in the diagnosis of coronary artery disease (CAD) and there are different imaging methods for screening and anatomical and/or functional assessment of CAD, however questions remain about the appropriateness and cost-effectiveness of screening of CAD together with the optimal approach to screening.
Echocardiography has been one of the dominant cardiac imaging modalities in patients with suspected cardiac disease, however, it has a very small value in the diagnosis and risk stratification of patients with suspected stable angina, since most of these patients have normal wall motion at rest, unless there is a history of prior myocardial infarction or myocardial stunning. Therefore, it will be beneficial if characteristic evaluated at rest can distinguish between a severe CAD from a less severe one39.
Global longitudinal strain (GLS) measured by 2-D speckle-tracking echocardiography (2-D STE) at rest has been recognized as a sensitive parameter in the detection of significant CAD, however, there is no conclusive evidence in the literature to support this. correlation between GLS values and myocardial ischemia evaluated with functional methods specifically with myocardial perfusion evaluated with SPECT technique.
In our study, we evaluated 40 patients of both genders, without comorbidities that could inherently affect GLS, with symptoms of stable angina or dyspnea who underwent cardiac SPECT to rule out ischemia and who had a transthoracic echocardiogram as part of their evaluation. at rest in which GLS was measured by 2-D speckle-tracking (2-D STE), they were divided into controls (n = 19) those with normal cardiac SPECT and cases (n = 21) those with positive SPECT for ischemia. Among the results we obtained, it stands out that 85.7% of the cases presented perfusion defects compatible with mild ischemia, 47.6% with moderate ischemia, and 13 subjects 61.90% presented perfusion defects compatible with infarction with mild ischemia in the residual tissue.
There were no subjects with severe ischemia or infarction with moderate or severe ischemia in the residual tissue, in the patients with ischemia the GLS was normal and there was no statistically significant difference between the controls.
According to the study carried out by S. Moustafa, et al., 200 candidates with suspected stable angina and conventional echocardiography at normal rest were evaluated, they underwent speckle tracking echocardiography and coronary angiography. Global and segmental longitudinal strain were evaluated and correlated with the results of coronary angiography in each patient, finding a statistically significant difference in the mean global longitudinal strain between normal coronary arteries and different degrees of coronary artery disease, and regional longitudinal strain (RLS) also showed statistical significance for the location of the affected vessel, concluding that two-dimensional speckle tracking echocardiography has good sensitivity and specificity to predict the presence, extent, and severity of CAD, showing an adequate correlation between GLS and regional abnormalities. and obstructive CAD, however it was a study where the presence of ischemia was not evaluated40.
These data agree with a systematic review carried out by I. Norum, et al., of the 781 patients included, 397 (60%) had CAD +. The mean global longitudinal strain was −17.2% (SD = 2.6) among the CAD+ patients versus -19.2% (SD = 2.8) in the CAD- patients. Study cut-off levels for CAD+ prediction in ROC analysis ranged from −17.4% to −19.7% with a sensitivity of 51% to 81% and a specificity of 58% to 81%. It is worth mentioning that RLS was not evaluated nor was ischemia sought41.
In the present study it was found that the global longitudinal strain (GLS) was normal in both groups, in the cases the GLS was −21 (range −15 to −29) and in the controls it was −21 (range −15.2 to −26), this shows that for the primary outcome, global longitudinal deformation is not affected in patients with stable angina and mild and moderate ischemia assessed by cardiac SPECT; however, the sample in this study included few patients and none with severe ischemia.
However, regarding the secondary outcome, it was observed that the regional longitudinal deformation was significantly reduced in the same region with perfusion defects in the cardiac SPECT, highlighting the inferior wall where the RLS was −21 in controls vs −22 (range −21 to −25.6) in cases with normal SPECT. In subjects with mild ischemia the RLS was −17.3 (range −16.8 to −19.1), in moderate ischemia it was −17.3 (Range −17 to −17.6.) and in subjects with myocardial infarction with residual light ischemia the RLS was −13.8 (−8.1 to −19.6) p. 0.0001.
In the study conducted by Dougdous, et. al., the relationship between CAD severity and resting three-dimensional speckle tracking echocardiography (3D-STE) was evaluated in patients with stable angina pectoris, including 120 patients with no history of CAD who underwent elective coronary angiography after of a positive stress test or positive myocardial perfusion SPECT. The mean RLS was −12, and a GLS value > −10 has a sensitivity of 88.9% and a specificity of 92.9% for detecting critical CAD, even though the subjects were evaluated with positive cardiac SPECT for ischemia before angiography, the degree of ischemia did not correlate with GLS values assessed by ultrasound (3D-STE)42.
Therefore, there is evidence that correlates reduced GLS and regional values with obstructive CAD, but not with ischemia assessed with non-invasive methods.
It is important to highlight that the previously mentioned data and those of the present study are applicable to patients with angina symptoms with stable evolution, LVEF > 50% and resting transthoracic echocardiogram without alterations in global and segmental mobility.
Conclusion
In the resting transthoracic echocardiogram, the longitudinal global deformation measured by 2-D speckle-tracking echocardiography (2-D STE) is not affected among patients with symptoms of stable angina and positive cardiac SPECT for mild and moderate ischemia. The regional longitudinal deformation evaluated by the same method shows a statistically significant decrease with respect to the segments without ischemia and correlates with the positive region for light and moderate ischemia in cardiac SPECT. The relevant findings of this study lead to new hypotheses that can be methodologically comparable through prospective cohort studies in order to evaluate the type of correlation between GLS, RLS, the presence and degree of ischemia, as well as with the findings of coronary anatomy evaluated by invasive angiography or coronary CT angiography in order to determine its predictive utility of GLS in ischemic heart disease in patients with stable angina.
Funding
The authors declare that this work was carried out with the authors’ own resources.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors have obtained approval from the Ethics Committee for analysis and publication of routinely acquired clinical data and informed consent was not required for this retrospective observational study.
Use of artificial intelligence for generating text. The authors declare that they have not used any type of generative artificial intelligence for the writing of this manuscript, nor for the creation of images, graphics, tables, or their corresponding captions.