Introduction
Meningiomas are the most frequent intracranial neoplasm of non-glial origin of the central nervous system, they are extra axial tumors originated from the meningo endothelial arachnoid cells present in the granulations of Pacchioni or arachnoid granulations, which consist of protrusions of the arachnoid membrane towards the interior of the dural sinuses that cross the holes in the dura mater. They are the most common primary tumors representing 36.4% of all cases, with an incidence of 7.7 per 100,000 inhabitants. Meningiomas are more frequent in women between the 4th and 6th decade of life under the influence of hormonal factors. In men they usually appear until after the 6th decade of life at the time when testosterone concentrations decrease1. They are usually benign neoplasms, however malignant strains have been described, among which the anaplastic and angiomatous variants stand out.
Clinical presentation
90% of meningiomas develop from the cranial meninges, the remaining 10% occur in the spinal meninges. Symptoms of clinical presentation reflect the anatomical region involved and compressed by the tumor or by peritumoral edema. The most frequent are: decreased visual acuity, trigeminal neuralgia, diplopia, and proptosis2,3, with a prevalence of 90%4. They are usually lesions of insidious onset due to their slow growth. They usually present intraorbital extension, which clinically manifests as hyperostosis that usually causes ocular proptosis due to the invasion of the orbital cavity space. Invasion of the dura mater usually extends to the lesser wing of the sphenoid, periorbita, temporal convexity and cavernous sinus. When invading the cavernous sinus there are usually also symptoms due to the involvement of cranial nerves II, III, IV, V and VI. These manifestations may be suggestive of malignancy, although in practical terms, most meningiomas are usually benign.
Diagnosis
The most useful tool for its diagnosis is still Computed Tomography (CT). It is usually superior to MRI to evidence the effects on the adjacent bone, especially the bone destruction that can appear in atypical and malignant meningiomas, or hyperostosis associated with benign meningiomas. Angiography is not mandatory for diagnosis; however, it is indispensable for surgical planning since meningiomas may be attached to large intracranial vessels or when preoperative embolization is planned to minimize blood loss during the surgical procedure.
Treatment
Neurosurgical resection by pre temporal craniotomy with supero latera orbitotomy, is the mainstay in treatment of this type of lesions. Subtotal tumor resection occurs in 10-80% of cases, with recurrence ranging from 8 to 58%5–8. Surgical treatment requires a delicate balance between performing complete resection of the tumor and avoiding irreversible neurological damage as a consequence of the surgical procedure itself. The incidence of postoperative neurological deficit varies between 2 and 30%9. and depends fundamentally on the location of the tumor and the extent of resection10. It is of vital importance to take into account that due to the nature of the meningioma and the surgical procedure aimed at resolving this type of tumor, the associated sequelae or complications are usually a crucial factor to consider, both from the functional point of view, aesthetic and reconstructive. The simultaneous skeletal reconstruction of the defect resulting from the resection of this type of tumor is a great challenge, to a large extent, due to the three-dimensional structure that they have, not only the bones that make up the roof, walls, and floor of the orbit, but their complex interrelationship to determinate the size and shape of the orbital cavity. Any error, however slight, when it comes to reestablishing the anatomy, will modify the especially important relationship of the orbital content with the skeletal continent. The main sequelae of suboptimal reconstruction are: Enolftamos, when there is hypocorrection, exophthalmos, when there is overcorrection, diplopia, when any of the extraocular muscles is trapped or does not have the appropriate excursion, orbital dystopia, among others.
So far, the reconstruction of these defects, when it has been attempted, has been very artisanal and has been based more on the experience of the reconstructive surgeon, than on objective elements. There are several resources availabe to perform the orbital reconstruction of the bone defect. Some examples are: calvary or rib bone grafts, methyl methacrylate, alloplastic porous materials, bioabsorbable meshes and titanium meshes, among others. Unlike other reconstructions of the craniofacial skeleton, there is no algorithm that could serve as a guide. Pritz and Burget in 200911 reported the use, in one case of a life-size sterolithography model where they created a mirror image of the healthy side as a model and anticipating the resulting defect, created a porous polyurethane implant, which was manufactured before surgery. They reported a decrease in surgical time and satisfactory outcome. Laroche et al., reported in 202212 the use of a custom-made porous titanium implant months before a tertiary reconstruction, using the patient’s CT scan and specialized software to recreate the zygomatic orbital frame and missing frontal bone after orbital exenteration, for recurrent meningioma. 3D model prints have been used as an aid for the management of complex fractures of the craniofacial skeleton, especially for pre-bending of titanium meshes and to reconstruct fractures with large defects in the orbial floor13,14.
After a case with these characteristics that resulted in a suboptimal reconstruction, it was decided to use virtual preoperative planning and build a three-dimensional model that could be useful, not only in the planning of the reconstruction, but also could be used and adapted during surgery to obtain a more anatomical reconstruction and thus reduce the postoperative sequelae. To our knowledge, this is the first time that this method has been used for the simultaneous reconstruction of defects caused by the resection of these tumors.
The main objective of this article is to evaluate whether using a life-size 3D model based on the CT scan of the patient, before and during tumor resection by the neurosurgeon, could result in more symmetrical and aesthetic results and with fewer postoperative functional sequelae, than when this tool is not used.
Material and methods
For the construction of the life-size 3D model, the patient’s CT scan an Ultra Print 3D software, a PHOTON MONO X resin printer and the assistance of a systems engineer were used. To achieve the desired versatility, the model was printed using Anycubic Plant Based UV resin. The cost of this process was $10,000 pesos (Fig. 1 and Fig. 2). The feasibility of manipulating the model and its resistance to usual sterilization techniques were verified before the first surgery.
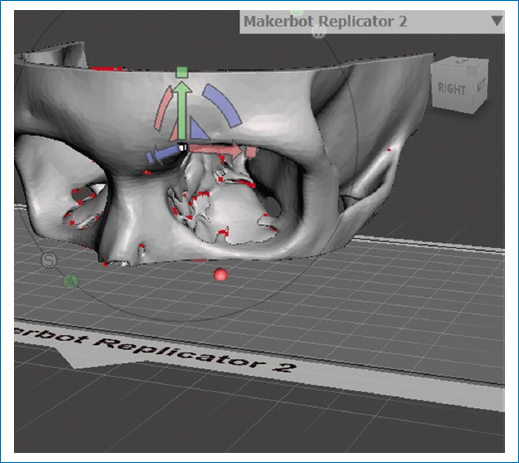
Figure 1. Using the CT scan to buil the life-size 3D model. Communication in real-time with the engineer Is used to achieve the exact replica.
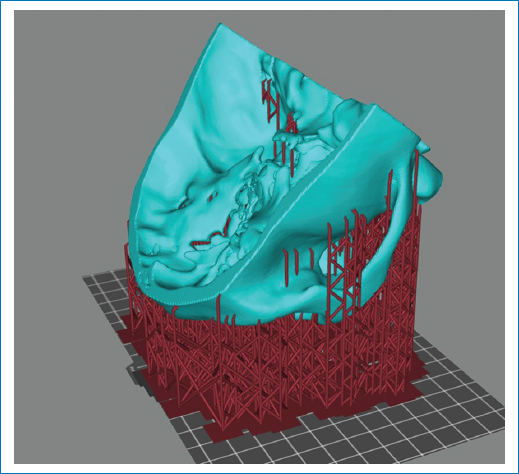
Figure 2. The life-size 3D model portrayed before printing.
Three consecutive cases reconstructed by the same surgeon, (the first two operated at the ABC Medical Center in Santa Fe and the third at the INNN), are presented. In the first one, a conventional reconstruction was performed without the aid of virtual planning or 3D printing of a model as a pre- and transoperative guide for the reconstruction of the skeletal defect resulting from tumor resection. In the last 2 cases, the method proposed in this article was used.
Case 1. A 40-year-old female patient who presents proptosis of the right eye of 2 months of evolution with rapid progression. Physical examination draws attention to the increased pressure of the right eyeball and the asymmetry between both ocular fissures. On the CT scan, a large exostosis of the right temporal bone as well as the roof and lateral wall of the orbit is evident. After the complete resection of the meningioma, reconstruction is performed using a titanium mesh, which rests on the entire floor and is given continuity to the margin that corresponds to the medial wall, trying to restore the normal anatomy of the orbital cone. In the postoperative period at 6 weeks, once edema resolved, right enophthalmos and lateral deviation of the ocular axis are evident (Fig. 3).
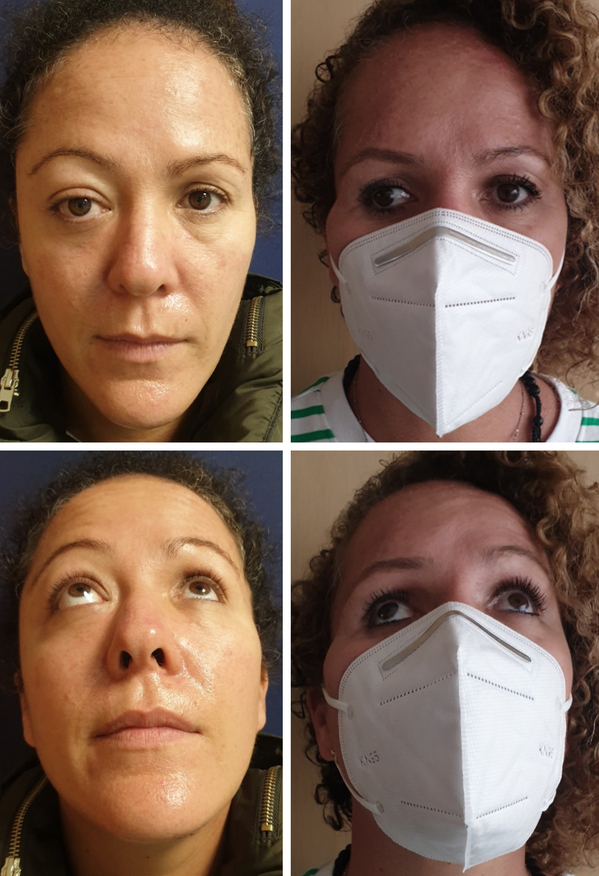
Figure 3. A and C: pre operative photos. Note exophthalmos and asymmetry in right supraorbital ridge, in addition to eyebrow dystopia. B and D: post operative at 6 months. Note right enophthalmos and ocular axis deviation.
Case 2. A 29-year-old male patient with left ocular proptosis of 10 months of evolution, that has been accentuating in the last 3 months and now is associated with visual problems (Fig. 4). In the CT scan (Fig. 5A) exostosis of the roof is evident with invasion of the orbital cavity that decreases its volume. Virtual planning was performed with 3D model printing and estimated tumor resection was discussed with the neurosurgeon (Figs. 5B-D). During the procedure, the model was used to achieve an exact reconstruction (Fig. 6 and Fig. 7).
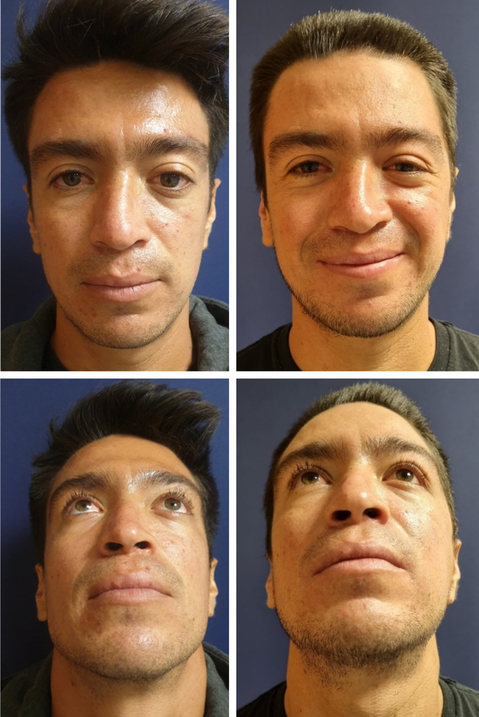
Figure 4. Pre A and C: and 6 weeks postoperative; B and D: using the proposed method. Note the total resolution of proptosis and symmetry.
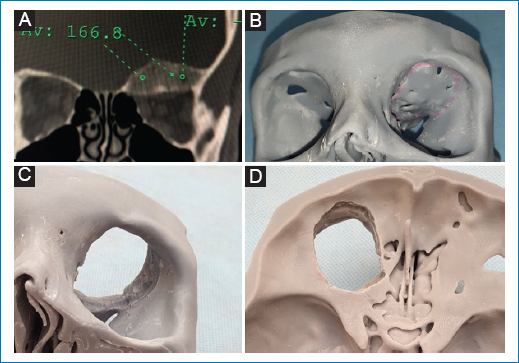
Figure 5. A: preoperative CT showing exostosis in roof and medial wall of orbit. Note how the volume of the cavity has decreased. B: model where preoperatively the resection of the neurosurgeon is estimated, before, C orbital view and D cranial base view after the supposed resection.
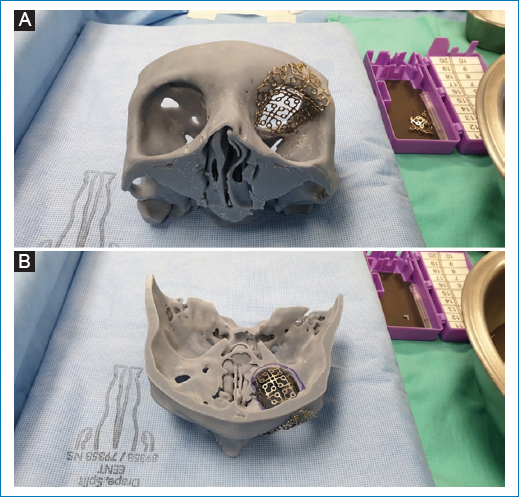
Figure 6. Once the neurosurgeon completes the resection of the tumor, using the model, the final margins are corroborated and custom modeling of the chosen reconstruction material is performed. In this case, titanium mesh. A: basal view. B: view of the craneal base. Note the importance of the model to recreate the 3D anatomy, guided by the unaffected side.
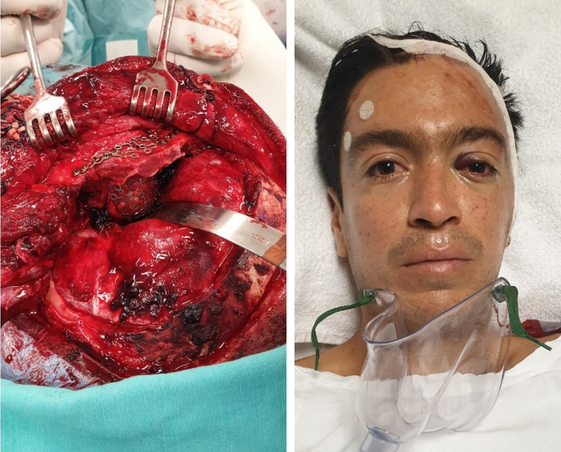
Figure 7. A: trans operative View from the craneal base. See how the mesh, molded to the exact size of the defect, restores the normal anatomy of the orbit (thick arrow) and the upper orbital rim (thin arrow) and separates its contents from the craneal base. The malleable separator retracts the temporal lobe of the brain to appreciate the depth of reconstruction. B: patient in the recovery room at the end of surgery.
Case 3. A 53-year-old female patient with a history of 2 previous attempts at meningioma resection invading the roof and lateral wall of the left orbit. As a result of two incomplete resections, severe left proptosis is evident. Total resection of the meningioma and guided reconstruction using 3D model was performed, with 2 titanium meshes, molded using the model to the exact size and shape of the defect. The postoperative result was very satisfactory (Fig. 8).
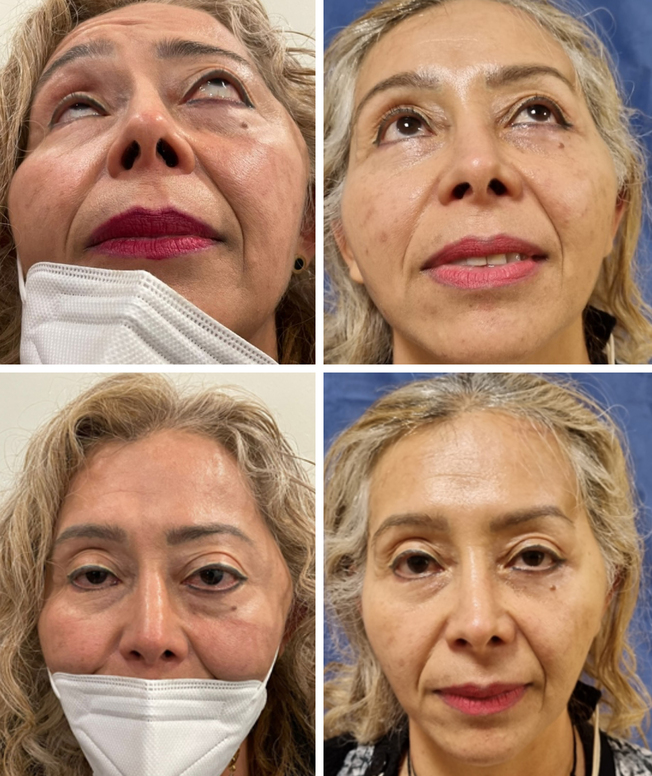
Figure 8. A and C: pre-operative photographs. B and D: result 1 year after reconstruction.
Discussion
The simultaneous reconstruction of the orbital walls and the greater and lesser wings of the sphenoid, especially with autologous bone grafts, has been promoted by some authors. The main advantage is to achieve a better aesthetic result, in addition to the prevention of postoperative complications such as pulsatile exophthalmos, fibrosis of the extraocular muscles, meningoceles and enophthalmos15–20. Whether with autologous or alloplastic tissue or with metal or absorbable meshes, oncological reconstruction of the orbit represents a formidable challenge. Not only due to the complex anatomical design of the orbit and the 7 bones that delimit it, but even more, due to the need to restore and respect the spaces contained therein, such as the superior and inferior orbital fissure and the optic nerve canal, and of course, not to injure the structures that pass through them. It is for all this that some authors question whether reconstruction is mandatory21. Being a benign neoplasm that usually affects patients of the 4th. decade of life, we believe that it is appropriate to try to avoid sequelae and at the same time improve the aesthetic result. Unlike other regions of the craniofacial skeleton, there is no algorithm, much less a guideline, for the reconstruction of these defects. So far, when these have been carried out, they have been based more on the experience of the surgeon and have been more artisanal than objective. In some cases, they went very well and in others they did not.
The tools associated with technological progress are currently a reality and an adjuvant of immense importance for craniofacial surgery, which allows the surgeon to perform surgical planning and simulation. In these cases, it was possible to generate real-size models based on the patient’s CT scan prior to the surgical procedure. Having the model printed in 3D, it was possible to plan the surgical resection of the meningioma, the defect that it was going to create, and finally, due to its composition, we were able to perform the final reconstruction in the model, during surgery. It is quite common in these cases that due to the actual extension of the tumor and the trans operative histological report, the margins of resection are increased, creating a 3D defect different and more extensive compared to the initial estimate. The model has the advantage of adapting during surgery according to the new margins, and in the same way, allowing us to adapt the titanium meshes to the final defect. In our hands this has enabled us to achieve functional and aesthetic better results in addition to significantly reduce surgical time. In the cases presented, orbital reconstruction was performed using titanium meshes due to the characteristics they possess, since they have a malleability that facilitates their incorporation into the orbital surface and adequate coverage by fibrous connective tissue a few days after the surgical procedure (15-48 days). Among the various advantages added to tissue incorporation, they do not stimulate an inflammatory process. Due to the fixation they require, it is difficult that they can migrate, they do not deform and do not interfere with tissue granulation or the recreation of native architecture. Nevertheless, the ideal material is autologous bone, it is extremely complex to adapt it precisely to the defect, its procurement has morbidity, increases surgical time and its success depends on obtaining total integration.
The time is not far away when it will be possible to create in bio reactors, hand in hand with technologies already used such as genetic engineering, the use of colonies of modified stem cells and 3D biological printers, original spare parts for complex missing structures. Meanwhile, we are convinced that it is not yet time to take the surgeon out of the equation, his experience, ingenuity, and ability to adapt to any scenario during such complex procedures.
Results
When virtual planning and a life-size 3D models were printed and used for reconstruction of the defects created after the resection of meningiomas that invade the orbit, it is our impression, that better aesthetic and functional results were obtained. No complication occurred with this reconstruction. Likewise, it was clear that the surgical time was reduced and surely, also of the costs of the reconstruction.
Conclusions
The new method proposed has allowed us to anticipate and above all, create a mental image with the three-dimensional characteristics of the defect resulting from the resection of the neoplasm, as well as to build in minutes, during surgery, a custom made spare part that in addition to restoring bone continuity, recreates the original shape of the orbital cavity maintaining the very important harmonic relationship between continent and orbital content, with very satisfactory functional and aesthetic results. It is necessary to extend this series and conduct a prospective study to analyze all the associated variables and thus make definitive conclusions.
Funding
The authors declare that this work was carried out with the authors’ own resources.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Use of artificial intelligence for generating text. The authors declare that they have not used any type of generative artificial intelligence for the writing of this manuscript, nor for the creation of images, graphics, tables, or their corresponding captions.