Introduction
Traumatic brain injury (TBI) is a public health problem affecting 2% of the world’s population, often resulting in some form of cognitive or motor disability. Motor disability, involving motor deficits, can be reversible or irreversible depending on the severity and location of the injury1,2.
After sustaining a traumatic injury, changes and alterations occur in the brain, including disruption of the blood–brain barrier, fluid extravasation, edema formation, inflammation1, diffuse axonal injury3, cytotoxicity4, fever5, and changes in neurotransmission, including systems such as the dopaminergic, GABAergic, and glutamatergic systems3,6,7. Furthermore, alterations in synthesis, release, and metabolism, as well as changes in transporter expression and receptors6–8, contribute to motor deficits9.
Fever has been described in both patients and trauma models, with increased body temperature after severe TBI being indicative of a grim prognosis. This complicates the management of febrile patients10, complicating outcomes and increasing the risk of mortality. Fever has been associated with metabolic changes and increased cerebral blood flow, inducing a thermogenic process11,12, which is compromised by the origin, extent, and severity of the injury having an impact on sequelae and the recovery process10,13. This is evidenced in ischemia studies, where an increase in temperature is observed in affected areas compared to intact brain tissue and body temperature12. There is also a relationship among the severity of intracerebral hemorrhage, increased temperature, and time since the injury14, as described in rats with severe trauma who exhibit hyperthermia for up to a week after the injury15.
However, in patients who survive a TBI, the biochemical and temperature changes sustained during the functional motor recovery process after sustaining the brain injury are not clear. This study evaluated whether increased temperature affects motor recovery and whether alterations in striatal GABAergic and glutamatergic neurotransmission are associated with functional motor recovery after a TBI-induced brain injury.
Method
Study subjects
A total of 28 male Wistar rats weighing 280 g were used from the UAM-Xochimilco animal facility, housed, and maintained in the LGII National Institute of Rehabilitation (INRLGII) animal facility in acrylic boxes with commercial rodent diet and water ad libitum, with 12/12-h light/dark cycles. All experimental protocols were approved by the INRLGII research and ethics committee (No. 74/23). Rats were handled following the Mexican Official Standard 062 ZOO 1999 and the Guide for the Care and Use of Laboratory Animals16. The number of animals used was kept to a minimum following festing statistical and bioethical criteria17.
Experimental design
Each rat was trained for 5 days to traverse a raised wooden beam, allowing evaluation of motor behavior through a rating scale and recording the degree of motor deficit. Once trained, all animals underwent daily baseline motor recording for 3 days, and body temperature recording as a reference before. After the injury, both recordings were performed for 20 days.
The animals were randomly categorized into 2 groups, sham and TBI, each with n = 14 and sub-classified into groups to measure gamma-aminobutyric acid (GABA) and glutamate release 3 and 20 days after the TBI (Fig. 1): (a) sham group (rats with surgical procedure but without TBI; day 3, n = 7; day 20, n = 7) and (b) TBI group (rats with severe TBI; day 3, n = 7; day 20, n = 7).
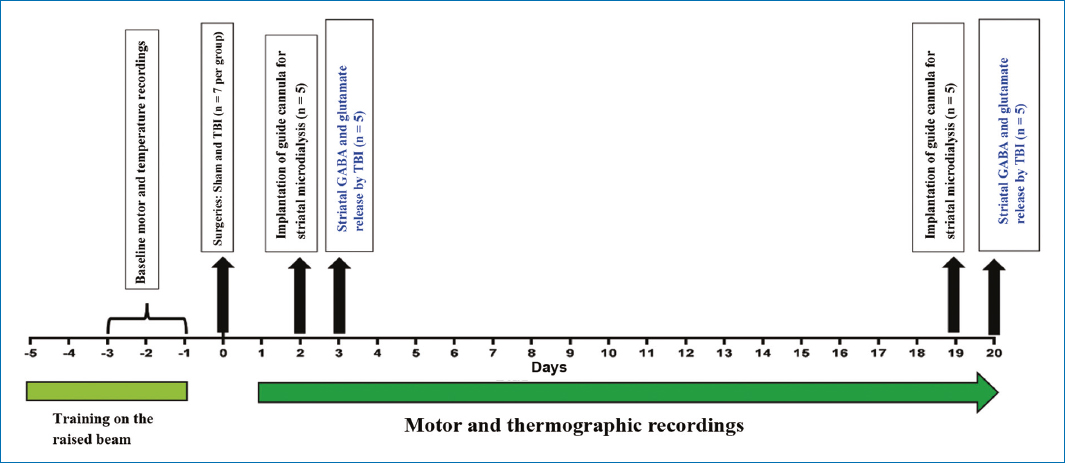
Figure 1. Experimental design describing the sequence over 20 days of motor and thermographic recordings, and surgical procedures, in each group.
Surgical procedures for cortical trauma injury and implantation of the striatal microdialysis guide-cannula
All rats were allocated to stereotaxic surgery. They were anesthetized with ketamine-xylazine (80-10 mg/kg) intraperitoneally and mounted on the stereotaxic equipment (Stoelting Co., Wood Dale, IL, United States). Body temperature was regulated and monitored using a CMA/150 temperature controller. Afterward, an iodine antiseptic solution was applied, and a cranial-caudal incision was performed; the skull was exposed, and a 1.5 mm diameter trephine was drilled using a hand electric drill (OSADA, XL-30W) at the level of the right primary motor cortex (M1), representing the left hind limb, according to pre-established coordinates (anteroposterior = +0.4 and lateral = −2.318, with respect to bregma). For the TBI group, the lesion was performed with an electromagnetic impactor (Impact One, Leica) using the following parameters: velocity 6, m/s; dwell time, 150 ms; and a 2 mm depth at the meningeal level, to induce severe cortical injury. Animals allocated to the sham group underwent the same surgical procedure described, but without traumatic impact.
During the surgical procedure, performed on day 0, the guide cannula was implanted in the striatum ipsilateral to the lesion. The coordinates for implantation were anteroposterior = +0.24, lateral = +2.8, and dorsolateral = 3.5, measured from bregma as a reference point. This procedure was performed to obtain dialysates 3 days after the injury. In the other group of animals allocated for day 20 after the injury, the implantation of the guide cannula was performed on day 19, and 24 h later, they underwent microdialysis in the striatum.
Each rat was sutured with non-absorbable silk thread gauge 2/0 with longitudinal needle (SC-26). Finally, buprenorphine 0.1 mg/kg was administered subcutaneously. Once recovered from surgery, rats returned to their cages and were provided with animal facility conditions.
Motor function analysis
The animals were evaluated and video-recorded every 24 h for 20 days after the injury using the balance beam test following the criteria described by Brailowsky et al.19 and modified by Gonzales-Piña et al.20. Motor deficit was assessed using a scale from 0 to 6 according to the following criteria: 0 = animals without apparent deficits; 1 = four toes of both hind limbs were off the edge of the beam; 2 = dragging of one hind limb (hypotonia); 3 = at least three slips or four toes of one hind limb off the beam; 4 = one fall or more than three slips; 5 = dragging of one or both hind limbs; and 6 = inability to walk. Scores were allocated in the four sections of the raised beam for a maximum possible score of 24 points. The videos were reviewed by an investigator blinded to the treatment conditions.
Temperature analysis
Animals were kept under controlled temperature conditions of 22°C with 55% humidity. For temperature recording, a FLIR E 50 camera equipped with a 18 mm FOL lens at a resolution of 240 × 180 pixels (emissivity = 0.95 and distance = 0.5 m) was used. Thermographic images were obtained of freely moving animals. Animals were placed on a flat surface and positioned at a mean distance of 20 ± 10 cm to capture the thermographic image, at the level of the external auditory meatus. Following this, each rat was returned to its cage to continue with the next animal. The images were analyzed using the FLIR Tools program tools to specifically select the area of the external auditory meatus. Thermograms were converted to a PDF file, from which temperature data were extracted and entered into an Excel database for further statistical analysis per day.
GABA and glutamate analysis in the striatum
To determine the concentration of GABA and glutamate release in the striatum ipsilateral to the injury on days 3 and 20 post-injury, each animal was placed in the freely moving microdialysis system, using a 4 mm length dialysis membrane. The probes were perfused with Ringer’s solution (mM: 146 NaCl, 4 KCl, 2.3 CaCl, and 2 mM phosphate buffer; the solution was adjusted to pH 7.4 with sodium hydroxide), using a CMA 400 perfusion pump (CMA/Microdialysis, Kista, Sweden). The flow rate was adjusted to 1 μL/min. Six fractions of the collected dialysates were obtained, with a final volume of 30 μL. Each fraction was collected every 30 minutes, and the system was stabilized with the first three dialysates, which were discarded.
For the separation of striatal GABA and glutamate, a high-performance liquid chromatography (HPLC) system was used employing a binary method (Alltech, two HPLC pumps, Model: 626). The HPLC system was coupled to a fluorescence detector from LINEAR, model FLUOR LC305. An Adsorbosphere OPA column of 100 × 4.6 mm internal diameter and 5 μm particle size from Alltech was used. A mobile phase of acetate buffer (using glacial acetic acid at 50 mM and adjusted to pH 5.9) and HPLC-grade tetrahydrofuran was used.
Statistical analysis
The statistical analysis of motor deficit scores was performed using the non-parametric Wilcoxon test to compare the average rank between the sham and the TBI groups. For biochemical and temperature analysis, the Student’s t-test was used. All values were expressed as mean and standard error. Differences observed in experimental conditions were considered statistically significant when results had p < 0.05.
Spearman correlation analysis was used to determine correlations between motor deficit and GABA or glutamate levels, as well as between temperature and motor deficit.
Results
Motor deficit and motor functional recovery
Results show motor deficit in the TBI group from day 1 to day 7 post-injury (p < 0.05). Each rat exhibited motor disability in the left hind limb. TBI group rats reached high scores on day 1, totaling 21 across the 4 sections of the raised beam, with a mean of 16; by day 2, they scored 15, with a mean of 10.8; and 7 days after the injury, the maximum score reached was 5, with a mean of 3.4.
Motor functional recovery was observed 7 days after the injury (Fig. 2). The TBI group exhibited the highest motor deficit 24 h after the injury, with a progressively, but significantly, reduced deficit that persisted until 7 days after the injury versus the sham group. Recovery was observed from day 8 onward and remained constant until the last recorded day, which was 20 days after the injury. On day 1 after the injury, the TBI group showed significant differences versus baseline recordings on days −3, −2, and −1, that is, before the injury, while the sham group on day 1 post-injury did not show significant differences versus their respective baseline values (p > 0.05).
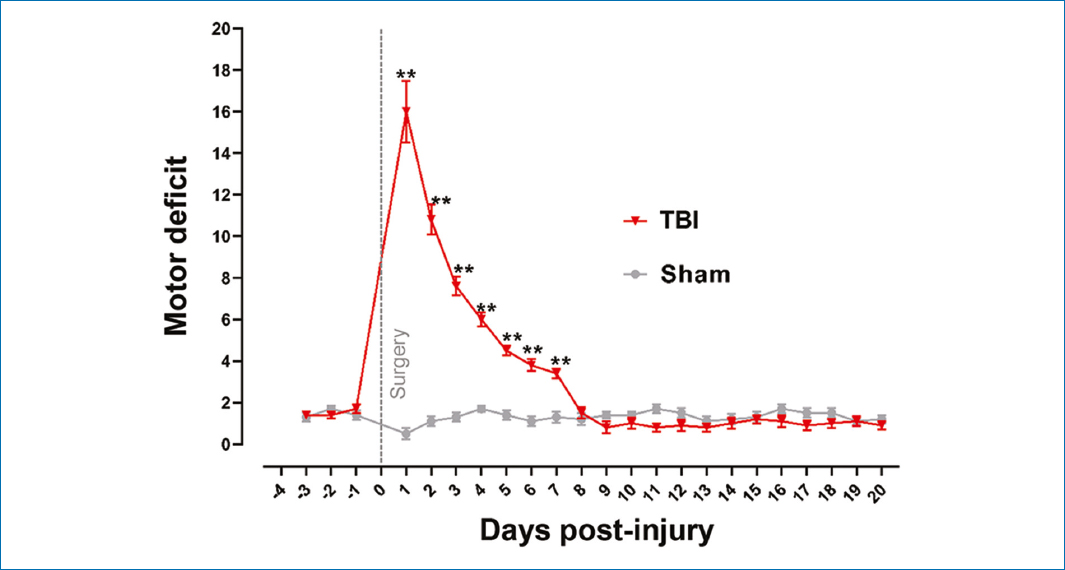
Figure 2. Record of motor deficit and functional recovery over 20 days following severe traumatic brain injury in the primary motor cortex (M1, representative of the left hind limb). Values are expressed as mean and standard error. Wilcoxon test was applied for statistical analysis to compare means between the TBI and the sham groups. **p ≤ 0.002.
Temperature analysis
Fig. 3 shows a statistically significant increase (p < 0.001) in temperature at the external auditory meatus in the TBI group within the first 4 days post-injury (p < 0.05) versus the sham group and their respective baseline on day −1. From day 5 onward, no significant differences were found versus the sham group, but days 5-8 showed differences versus their respective baseline on day −1. Temperature normalization was observed 9 days after the injury versus the baseline recording on day −1. The sham group exhibited an increase in temperature within the first 2 days post-injury (p < 0.001) versus their respective baseline on day −1.
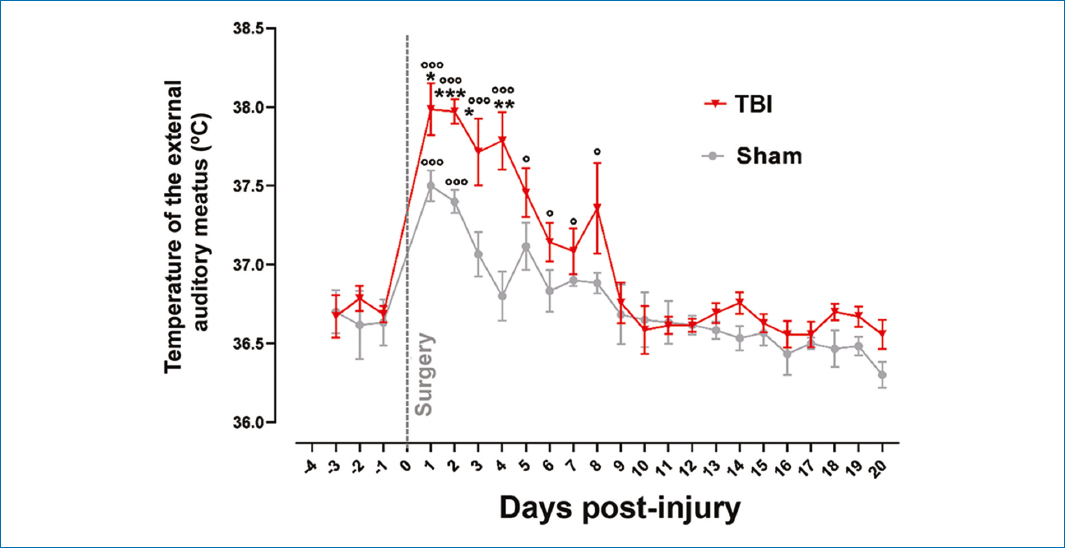
Figure 3. Records of temperature at the level of the external auditory meatus, baseline values, and their evolution after severe traumatic brain injury. Values are expressed as mean and standard error. Student’s t-test was used for statistical analysis to compare means between the TBI and the sham groups. ***p < 0.001 and versus their respective baseline values; ooop < 0.001; op < 0.05.
The TBI group exhibited mean temperature values of 37.8°C within the first 4 days post-injury, with a difference of 0.5 and 1.0°C versus the sham group (p < 0.001 for days 2 and 4, and p < 0.05 for days 1 and 3).
In addition to measuring temperature at the external auditory meatus, tail temperature was analyzed as an indicator of thermoregulation in rats. Traumatic cortical injury produced changes in tail temperature versus the baseline recording on day −1, indicating that the injury did not alter thermoregulation (Fig. 4).
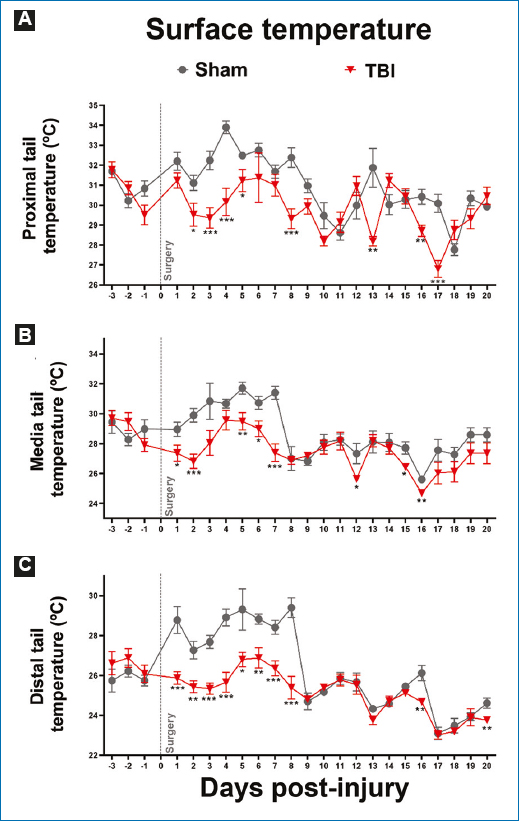
Figure 4. Records of tail temperature in its A: proximal, B: middle, and C: distal sections, describing baseline records and their evolution after severe traumatic brain injury (TBI). Values are expressed as mean and standard error. Student’s t-test was used for statistical analysis to compare means between the TBI and the sham groups. ***p < 0.001; **p < 0.01; *p < 0.05.
In the proximal region of the tail, 24 h after the injury, the TBI group did not show statistically significant differences versus the sham group (p > 0.05). However, from day 2 to day 5, a lower temperature was observed in the TBI group versus the sham group (p < 0.05).
On day 4, the sham group recorded the highest temperature, at 34°C, and on day 18, the lowest temperature was recorded, at 27°C. The sham group experienced peaks and troughs in temperature, but the latter were not as low as in the TBI group. This group reached the highest temperature 6 days after the injury, exceeding 31°C, while the lowest temperature was recorded on day 17 at below 28°C. On precisely that same day 17, the last significant difference was recorded versus the sham group (p < 0.001).
A similar pattern to the temperature recorded in the proximal tail was observed in the middle and distal regions of the tail, both in the TBI and sham groups. As already mentioned, despite temperature variation on different recording days in the three tail regions evaluated, no statistically significant differences were found versus their respective baseline recordings (Fig. 4). In addition, analyzing the relationship between changes in temperature at the external auditory meatus and motor deficit after TBI revealed that body temperature changes showed no correlation with motor deficit evaluated on day 3 (r = 0.57; p = 0.257), nor with observed motor functional recovery on day 6 (r = 0.038; p = 0.952).
Analysis of GABA and glutamate release in the striatum
GABA and glutamate release was assessed using microdialysis and HPLC techniques. Fig. 5A shows a 33% reduction in GABA concentration in the TBI group versus the sham group on day 3 post-injury (p < 0.01). This reduction was restored by day 20 post-injury, reaching concentrations similar to those of the sham group (Fig. 5B). Regarding glutamate release, a 47% reduction was seen in the TBI group versus the sham group on day 3 post-injury (Fig. 5C); this concentration was restored to levels similar to those of the sham group by day 20 post-injury (p > 0.05) (Fig. 5D). Furthermore, analyzing the relationship between changes in striatal GABA and glutamate and motor deficit after TBI revealed that the reduction in released GABA and glutamate concentrations showed no correlation with motor deficit evaluated on day 3 (r = 0.105, p = 0.867; r = 0.790, p = 0.133, respectively). Similarly, motor functional recovery after injury showed no correlation with GABA and glutamate concentrations on day 20 (r = 0.948, p = 0.167; r = 0.223, p > 0.999, respectively).
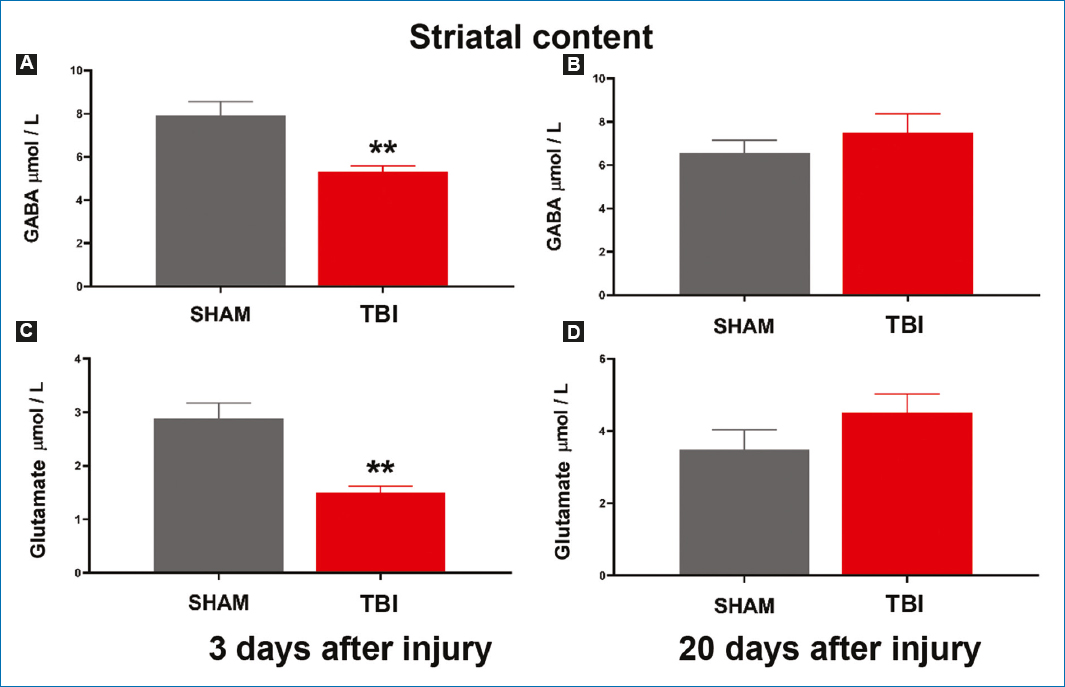
Figure 5. Concentrations of GABA and glutamate released in the homolateral striatum to the injury. GABA concentration A: 3 and B: 20 days after the injury. Glutamate released concentration C: 3 and D: 20 days after the injury. Values are expressed as mean and standard error. Student’s t-test was used for statistical analysis to compare means between the traumatic brain injury and the sham groups. **p < 0.01.
Discussion
In this study, we found that severe TBI in the right cerebral hemisphere, at the level of the primary motor cortex affecting the left hind limb, results in a motor deficit that persists within the first 7 days post-injury. This timeframe is similar to that reported in studies of FeCl221 induced and cortical ablation-induced brain injury22. We observed that the greatest motor deficit occurred 24 h post-injury and functional motor recovery began from 192 h onward, which is consistent with the findings made by Ávila-Luna et al.22 in another study. Trauma-induced motor deficit is associated with cortical neuron death due to mechanical injury, and axonal injury affecting subcortical structures such as the basal ganglia20, nuclei partly associated with motor function23, and the thalamus, associated with body temperature regulation24. Their disruption promotes motor deficits23,25, caused by inhibition and changes to the cortico-striatal-thalamo-cortical pathway6,23,26,27, and changes to neurotransmitter release and metabolism3.
Motor deficits following brain damage are reversible sequelae, depending on type, location, and severity, as demonstrated by rehabilitation patients achieving motor functional recovery after brain injury1,2. In this study, functional recovery occurred 8 days post-injury, possibly related to reactivation of inactive molecular and cellular processes. It has been suggested that this involves activation of cell genesis and repair, changes of existing neuronal pathway properties, and activation of neuroanatomical plasticity, leading to formation of new neuronal connections28 and implying a process of relearning29.
In this study, we demonstrated an increase in body temperature, assessed at the external auditory meatus, following TBI, as also described by Sazbon and Groswasser30, who report an incidence rate of neurogenic fever in trauma patients ranging from 4% up to 37%. We noted that this temperature increase persisted within the first 192 h post-injury and returned to baseline values by day 9 post-injury. It has been described that individuals with TBI are at risk of fever unrelated to infectious etiology, where body temperature adjustment is impacted by endogenous substances such as pyrogens, interleukins 1 and 6, tumor necrosis factor, and interferon, which, in turn, increases prostaglandin levels. This leads to hypothalamic thermoregulatory activation31–33.
Furthermore, we reported that the surgical procedure per se in non-injured animals did not generate any motor deficits, as described by Zhao et al.34, but induced a temperature increase within the first 48 h after surgery. However, this temperature increase did not compare to that seen due to TBI. Reports describe that post-operative temperature can rise due to increased interleukin 6, a proinflammatory protein that stimulates antibody production, resulting in mild fever. This, along with other factors such as stress from sedation effects and tissue injury induced by incision, promoting vascularization and inflammation mechanisms, results in temporary fever spikes33.
Although an increase in body temperature recorded at the external auditory meatus was observed during the recording days, no changes in thermoregulation were seen, assessed using thermographic records on the animals’ tails. It has been described that the rat tail acts as a thermoregulatory organ35. Although temperature variation occurred after surgical intervention and injury, no significant differences were seen between the TBI and sham groups. This variation can be associated with increased temperature caused by anesthesia. It is documented that the rat tail exhibits vascularization for heat dissipation, and its function is demanded by brain injury. This leads to temperature drops within the first 3 days, which are attributed to damage. Temperature normalization would be related to the involvement of adipose tissue, as described by Laird et al.36 in their study.
We should mention that the temperature increase observed in injured animals in this study is consistent with episodes of mild fever and does not compromise the subjects’ lives. In addition, we demonstrated that this temperature increase is not associated with motor deficits or motor functional recovery.
Former studies have shown that changes to glutamate and GABA levels during acute and chronic periods may compromise patient life after brain injury7,37. In our study, we reported a reduction in GABA and glutamate levels in the homolateral striatum at 3 days, with restoration during the motor recovery period. The reduction in glutamate and GABA levels could be associated with altered corticostriatal pathway connectivity and decreased activity of GABAergic striatal neurons27,38,39. Restoration of glutamate and GABA levels could be related to neuroplasticity mechanisms involving inactive molecular and cellular processes. It has been suggested that activation of genesis, cell repair, changes of existing neuronal pathway properties, and activation of neuroanatomical plasticity lead to formation of new neuronal connections28.
Conclusion
Our study provides significant evidence on the complex biological interactions occurring after TBI. We demonstrated an increase in body temperature at the external auditory meatus, which extends within the first 192 h post-injury, indicating a disruption in thermoregulation. However, this change does not appear to be associated with motor deficits or motor functional recovery. In addition, we observed critical changes in glutamate and GABA levels in the homolateral striatum, which initially decrease after injury and then recover during the motor recovery period. These findings suggest a change and subsequent recovery in corticostriatal pathway connectivity, possibly due to neuroplasticity mechanisms.
This research expands our understanding of physiological and biochemical responses following TBI, providing a basis for future studies on therapeutic interventions in this area.
Funding
The authors declare that they have not received funding.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article. Furthermore, they have acknowledged and followed the recommendations as per the SAGER guidelines depending on the type and nature of the study.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Use of artificial intelligence for generating text. The authors declare that they have not used any type of generative artificial intelligence for the writing of this manuscript, nor for the creation of images, graphics, tables, or their corresponding captions.