Introduction
The Jornadas Quirúrgicas IMSS Bienestar (formerly known as Surgical Medical Encounters) is an institutional strategy, initiated in 1996, that provides highly specialized medical services to the population in marginalized rural areas. They consist of volunteer and altruistic medical specialists who support the country’s most vulnerable population with the aim of providing highly specialized medical care to the pediatric population. We established the area of pediatric reconstructive surgery in 2009 to address congenital and acquired problems where there are no highly specialized medical hospitals. The main conditions among the pediatric population within this program are primary and secon- dary cleft palate, microtia, other auricle deformities, and congenital hand deformities. All, except for ear deformities, have surgical treatment guidelines in Mexico.
This paper presents the first report of a comprehensive care program for auricular deformities in rural hospitals. We propose an innovative and reproducible surgical technique performed in two surgical stages. This technique is carried out in High-Specialty Medical Units within the Instituto Mexicano del Seguro Social (IMSS).
Microtia is defined as a malformation of the external ear characterized by a small auricle, which includes a broad clinical spectrum of presentation, from minor anomalies to total absence of the auricle1. Its presentation is a public health problem due to its high prevalence and the psychosocial sequelae experienced by patients2.
Abnormal development of the 1st and 2nd branchial arches, the branchial cleft, and the pharyngeal pouch produces malformations of the external and middle ear. Those limited to the auricle require reconstructive corrections3. The external auditory canal derives from the first branchial cleft and is represented by a core that remains in place until the 7th month of gestation, at which point the middle and inner ear are already formed. Resorption of epithelial cells begins, and if this process stops prematurely, there will be a normal tympanic membrane and bony external auditory canal, but the cartilaginous portion will be atretic or stenotic. The medial portion of the external auditory canal is formed by the bones of the eardrum4. The auricle forms from six auricular hillocks originating from the tissue of the 1st and 2nd pharyngeal arches. These surround the pharyngeal cleft and contribute to specific components of the auricle itself. Different signaling molecules and proteins are involved in the morphogenetic and differentiation processes of the auricle5,6.
The incidence rate of external and middle ear malformations is 1 in 10,000 or 20,000 live births, occurring more frequently in Latin America. Population studies conducted in some European countries and the United States show a prevalence between 0.83 and 4.34/10,000 births. In the United States, ethnic variations have been reported, with a higher prevalence among individuals of Japanese Asian origin and in the Latin American population; in the Navajo Indian population, a prevalence of 1 in 1200 has been reported7,8. In Chile, the reported frequency is 4.4/10,000 live births. In Mexico, the Registry and Epidemiological Surveillance of External Congenital Malformations has reported a prevalence of 7.37/10,000 births2,9,10. In tertiary referral centers, this malformation is among the leading causes of outpatient visits. From 2006 through 2010, 499 cases were treated at the Hospital Infantil de México, and 318 cases at Hospital de Pediatría Centro Médico Nacional Siglo XXI from 2002 through 2006. At Instituto Nacional de Rehabilitación, a total of 149 cases have been reported12. Microtia occurs more frequently unilaterally (79-93%) and on the right side (60%). It predominantly affects men and is associated with atresia or stenosis of the external auditory canal (55-93%). More than 80% of patients present with conductive hearing loss on the affected side13–16.
In 1926, Hermann Marx published the first classification system for congenital anomalies of the external ear17. Since then, different classifications have been proposed based on the surgical and embryological aspects of the deformity18. Eavey describes four degrees based on the severity of auricular presentation19. Most clinicians use the Hunter classification system20 ranging from a small auricle to anotia. In 1975, Tanzer published one of the most widely used classifications in reconstructive surgery, as part of the clinical and surgical approach to congenital ear deformities21.
Auricular reconstruction, regardless of the type and associated deformity, requires two elements: the autolo- gous costal cartilage framework with projection and skin coverage22. A variety of surgical techniques are reported for auricular reconstruction, each composed of several stages depending on the severity, size, position, quality of the cartilaginous remnants, and the surgeon’s preferences23,24.
Auricular reconstruction techniques propose a 3D projection that includes the tragus and the depression for the concha25,26. Most techniques require > 2 surgical stages of reconstruction, with subsequent touch-ups. Key points for reconstruction are an appropriate plan, attention to framework details, adequate dissection of the skin flap, and proper positioning of the cartilaginous framework27,28.
Total auricular reconstruction is one of the most difficult procedures for reconstructive surgeons. Although knowledge of current techniques provides an encoura- ging start to treating this problem, results are not always reproducible, which decreases interest in venturing into this type of surgery. The incidence rate of auricular deformities in the hospitals where we work is high, which motivated us to create a surgical technique that could be reproducible and provide optimal results in less time. Using the costal synchondrosis of ribs 6 and 7 contralateral to the auricular defect, we formed a cartilaginous framework with a 3D structure, projecting the most important ear structures, the helix and antihelix, and deepening the conchal area, similar to the porous polyethylene implants used by Reinisch29, from which the idea for our framework for auricular reconstruction emerged.
We propose an innovative surgical technique for the treatment of microtia and other auricular deformities in two surgical reconstruction stages. After stage #1, we did a 6-month follow-up, evaluating the reconstructed structures and scheduling the second stage of auricular reconstruction at that point.
Surgical technique
STAGE 1 OF RECONSTRUCTION
- – Retrolobular incision, directed toward the continuity of the neohelix position, with a length equal to the auricular lobule located anteriorly and preauricularly (Fig. 1).
- – Dissection of the retroauricular skin flap, with a limit of 5 mm beyond the pre-auricular hairy area.
- – Rapid intraoperative tissue expansion of the skin flap using an 18-FR Foley catheter balloon. This is achieved by performing only the retrolobular approach to keep the catheter in place while gradually inflating the balloon with injectable solution.
- – Hemithorax approach for costal synchondrosis harvest from the 6th to 7th intercostal space contralateral to the auricular deformity (Fig. 2).
- – Meticulous dissection, opening the fascia of the rectus abdominis muscle without incising the muscle. The muscle is dissected along its fibers by blunt dissection only to separate it.
- – Opening of the perichondrium for harvesting the 6th and 7th costal synchondrosis en bloc, from the costochondral junction to the sternum (Fig. 3).
- – Harvesting of the costal synchondrosis block. To assemble the framework, we rotate the block, changing its convexity to concavity. In this way, when rota- ting the 7th synchondrosis, which is the longest, it will be carved as the helix. From the 6th synchondrosis, we will form the antihelix, superior and inferior crura, and the intertragal incisure, projecting the antitragus, all as a single piece. The structures are joined with 3/0 non-absorbable monofilament suture (Fig. 4).
- – For the introduction of the cartilaginous framework, we continue the retrolobular incision along the pre-auricular skin remnant corresponding to the lobule.
- – All cartilaginous remnants underneath the pre-auricular skin lobule are dissected with a blunt technique, which will allow deepening of the area corresponding to the neoconcha. The temporal artery runs underneath these remnants, so extreme caution is advised (Fig. 5).
- – Before introducing the cartilaginous framework, we re-evaluate the expanded skin pouch, meticulously check hemostasis of the area, and place a drain, preferably a 10-Fr medical-grade silicone drain with a 100 cc round suction reservoir or an 8-Fr feeding tube, applying negative suction with a 10 cc syringe. The drain is exteriorized through a counter-incision toward the lower retroauricular area and fixed with a 3/0 non-absorbable monofilament suture. This drain is removed after 5 days.
- – The cartilaginous framework is introduced into the auricular area, guiding the inferior crus toward the tail of the eyebrow, which will naturally and appropriately position the framework.
- – The lobule is then rotated, providing continuity to the helix, and the skin is sutured with 4/0 non-absorbable monofilament in a simple running stitch. Excess skin is resected, and it is assessed that there is no air leak to achieve adequate adhesion of the skin flap to the cartilaginous framework structures. We cover only the surgical approach wound with gauze, and the formed framework is covered with adhesive plastic; this allows monitoring post-operative bleeding and ensuring that the definition of the structures is not lost.
- – The donor area in the chest is closed in layers after a Valsalva maneuver to assess pleural integrity. We administer 100 units of botulinum toxin type A to the rectus abdominis muscle to relax it in the surgical area30, and block intercostal nerves with ropivacaine, which has a long-lasting anesthetic and analgesic effect. The aponeurosis is sutured with 3/0 absorbable monofilament of glycolide-epsilon-caprolactone copolymer with simple stitches, and the skin with the same 4/0 suture caliber using intradermal and subdermal stitches.
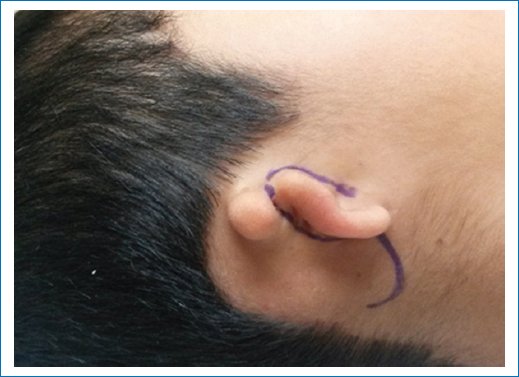
Figure 1. Marking of retrolobular incision.
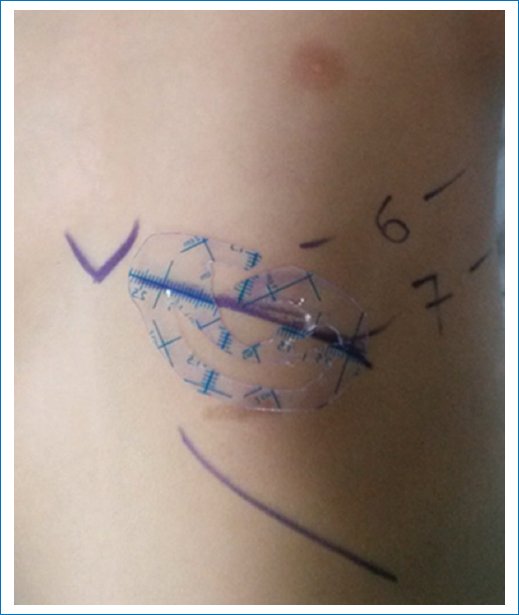
Figure 2. Marking for harvesting the 6th and 7th synchondrosis, a plastic mold in the dimensions of the healthy ear.
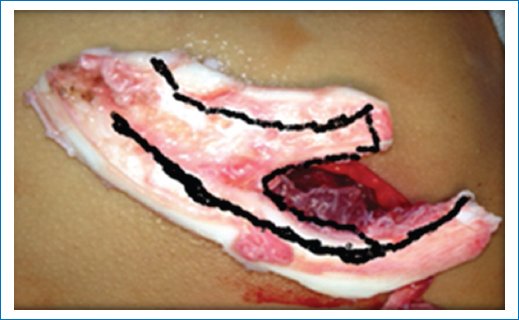
Figure 3. Block of costal synchondrosis for the formation of the cartilaginous framework.
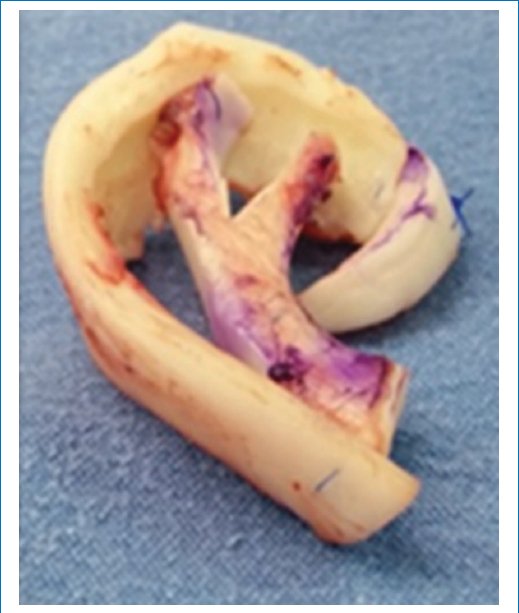
Figure 4. Formation of the 3D framework.
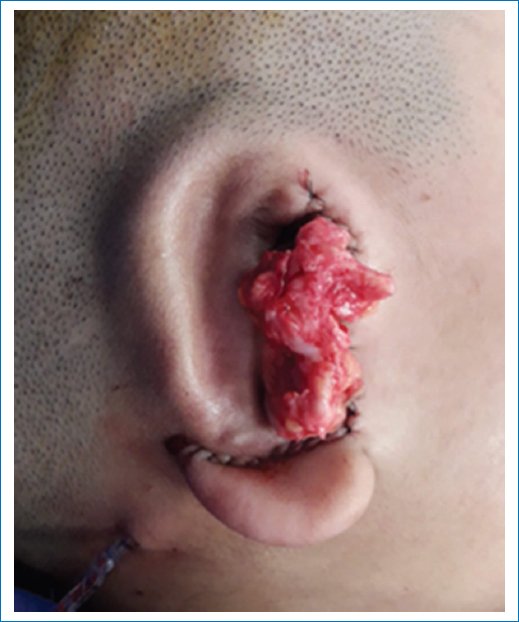
Figure 5. Resected cartilaginous remnants to deepen the neoconchal area.
STAGE 2 OF RECONSTRUCTION
Six months after the first reconstruction stage, we perform the opening of the auriculocephalic sulcus.
- – Approach 3 mL from the helix contour of the formed framework, following hydrodissection with lidocaine with epinephrine. The incision is performed from the middle of the helix root contour to the lobule of the cartilaginous framework (Fig. 6).
- – Dissection of the retroauricular skin flap toward the temporoparietal area, which will provide space for the dissection and elevation of the cartilaginous framework. Exposure of the cartilaginous framework should be avoided. The superficial temporal fascia will be the base for lifting the framework, and the deep temporal fascia will be the retroauricular area.
- – The retroauricular flap with temporoparietal dissection is advanced, fixing it to the deep temporal fascia with a 3/0 absorbable monofilament suture of glycolide-epsilon-caprolactone copolymer using simple stitches. The resulting rotation flap from the advancement is resected and sutured with the same suture using a simple running stitch. The goal is to reduce the area to be grafted in this region.
- – The dimension of the raw retroauricular area and the posterior area of the framework is considered to design a full-thickness skin graft from the ipsilateral inguinal region. The donor area is closed with 4/0 absorbable monofilament suture of glycolide-epsilon-caprolactone copolymer using intradermal and subdermal stitches.
- – The skin graft is fixed with 4/0 non-absorbable monofilament suture using a simple running stitch. The auricular lobule is aligned with the framework, providing adequate continuity to the helix, sometimes requiring an asymmetric Z-plasty to achieve continuity of the structures. It is sutured with 5/0 non-absorbable monofilament.
- – A bolster is placed to allow adequate adhesion of the graft to the raw area, which is then fixed with 3/0 non-absorbable monofilament suture. A head bandage is applied, covering the reconstructed area with gauze pads to form a cushion.
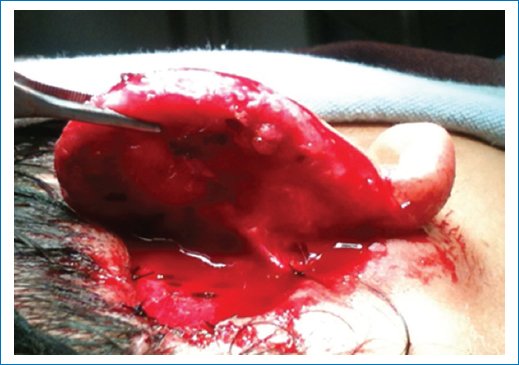
Figure 6. Opening of the auriculocephalic sulcus.
Material and methods
We conducted a descriptive, observational, retrospective study to document patients who underwent auricular reconstruction during Jornadas Quirúrgicas IMSS Bienestar, from the second half of 2009 to the second half of 2023. For each surgical journey site (Rural Hospital), the following variables were considered: number of patients operated, age, sex, diagnosis using the Tanzer classification, side of the auricular defect, number of stage #1 and stage #2 surgical procedures performed, whether treatment was completed with follow-up (attending follow-up for evaluation of stage #1 reconstruction outcome), and complications presented from the stage #1 reconstruction of the reconstructed area and the donor area. (Data referred to in the surgical journey activity and follow-up reports submitted to the IMSS Special Health Projects Coordination). Through informed consent and authorization of the surgical procedure, approval for the surgical procedures was obtained, validated by the Rural Hospital’s teaching committee, in full compliance with the current standard for health records.
Results
Over 14 years, 30 surgical journeys have been conducted since 2009. Auricular reconstruction procedures using the previously described innovative surgical technique were documented. A total of 22 follow-ups of these journeys were performed to complete the reconstruction stages, providing comprehensive care.
A total of 268 patients were operated on, 183 of whom were men (68.28%) and 85 were women (31.71%). The mean age was 10 years (range from 6 to 18 years). By diagnosis, using the Tanzer classification, microtia was the most frequent auricular malformation (Table 1).
Table 1. Frequency of auricular malformation
| Tanzer classification | Frequency |
|---|---|
| I. Anotia | 12 |
| II. Microtia | 210 |
| III. Hypoplasia of the middle third of the ear | 12 |
| IV. Hypoplasia of the upper third of the ear | 23 |
| V. Prominent ear | 11 |
Regarding the location of the auricular defect, the right side was most affected in 153 patients (57%), the left side in 84 (31.34%), and bilateral in 31 (11.56%).
A total of 229 patients diagnosed with microtia and other auricular deformities were operated on using our innovative technique, harvesting only the 6th and 7th costal synchondrosis with lobule rotation in stage 1.
A total of 23 patients required otoplasty surgery (11 diagnosed with prominent ears and 12 with hypoplasia of the upper third, retracted ear modality); 16 patients underwent another technique to treat their auricular problem, 11 of whom had a past medical history of auricular reconstruction with the Brent technique, thus stage 2 corresponding to lobule rotation was performed.
Of the 229 total auricular reconstructions performed, 164 patients, or 71.6%, completed their reconstruction with the opening of the auriculocephalic sulcus.
Complications reported in stage 1 of reconstruction occurred in six patients, which corresponds to 2.8% of the total operated patients. Four complications in the reconstructed auricular area, and two in the costal graft donor area.
Discussion
The results obtained are consistent with reports from similar publications; microtia occurs more frequently unilaterally and on the right side. In this study, we considered all reported auricular deformities based on the Tanzer classification. The most affected side is the right (58.4%), predominantly occurring in men (68.60%).
The Brent technique for microtia reconstruction was published in 1974 and is performed in four surgical stages. Stage 1 involves harvesting the 6th, 7th, and 8th contralateral costal synchondroses and subcutaneous placement of the carved cartilaginous framework through an anterior auricular incision. Stage 2, 3 months later, involves lobule rotation. Stage 3 is the opening of the auriculocephalic sulcus with elevation of the cartilaginous framework. Reconstruction stage #4 involves deepening the concha and grafting for the tragus. Long-term results with this technique are successful with a minimal rate of infection, extrusion, and hematoma. In his study of 600 cases of auricular reconstruction, Brent reports common complications such as pneumothorax, infection, cartilage framework exposure, lobular necrosis, and changes in framework size. Overall, the ear cartilage structure will grow with the patient22.
Satoru Nagata published a new method of auricular reconstruction in two surgical stages in 1993. To achieve the 3D appearance of the framework, he harvests four costal synchondroses, and the projection of the framework is achieved by placing a graft underneath the framework, requiring a temporal fascia flap. This technique improves conchal depth and helix root projection31. Using Nagata’s technique, Firmin reports a 14% incidence rate of skin flap necrosis32.
In Mexico, Dr. Fernando Ortiz Monasterio33 and Dr. Sergio Zenteno Alanis34, pioneers in auricular reconstruction, share their experiences to establish a standardized management for patients with this craniofacial alteration. Costal synchondrosis harvest is the common denominator for reconstruction.
Only 6 (2.8%) of the 212 patients operated on with this innovative technique exhibited complications in the reconstruction stage 1. Four were in the reconstructed auricular area: three cartilage extrusions, with two frameworks being removed due to infection. Moreover, one framework was salvaged by an emergency superficial temporal fascia flap and covered with a skin graft. One patient exhibited lysis of the skin flap in the neoconchal area, requiring a full-thickness skin graft. From the donor area, two complications were reported, corresponding to pneumothorax: in one case, the pleural lesion was repaired intraoperatively, and another patient required the placement of a water seal in the immediate post-operative period. No thoracic deformity was reported; this is because only two synchondroses are taken, unlike the Brent and Nagata techniques, which require more cartilage to create the framework. With this technique, there is no instability of the rib cage, which has allowed us to perform total bilateral auricular reconstruction in three patients, a treatment not reported in the literature.
This technique can be considered in the Mexican surgical treatment guidelines for the surgical treatment of microtia and other deformities such as anotia, mid and upper third hypoplasia. This technique, which we use at Hospital de Pediatría del Centro Médico Nacional Siglo XXI, has reduced reconstruction times and decreased surgical deferrals, providing high-quality care with optimal and reproducible results.
Conclusion
Auricular reconstruction is one of the most challenging surgical procedures for the reconstructive surgeon. Achieving optimal results is the goal to provide the patient with physical and psychological integrity that allows their integration into society.
To date, the use of costal cartilage for auricular reconstruction remains one of the most durable and ubiquitous techniques for microtia reconstruction, providing excellent and long-lasting esthetic results.
Due to its presentation in our country’s population, it is considered a public health problem.
Costochondral grafting is the best option for surgical treatment. Most current surgical techniques require biological, artistic, and mathematical management to carve a cartilaginous framework that resembles the healthy contralateral ear. We publish an innovative and reproducible surgical technique that offers optimal results.
By harvesting the 6th and 7th costal synchondrosis as suggested, we structure a 3D framework. Complementing the technique with the resection of cartilaginous remnants and repositioning of the auricular lobule allows for an optimal result in the projection and concavity of the ear structures to be reconstructed.
A total of 162 patients (76.4%) out of the 212 recons- tructions performed with this new technique completed their treatment with reconstruction stage #2. Some patients’ parents did not accept the reconstruction stage #2, as their expectations for shape and projection were met with the first surgery. The complications reported in this series are minimal for both the donor area and the reconstructed area. The reproduction of this technique within the reconstructive surgical community is possible, as artistic ability is not required; careful tissue management is a prerequisite for achie- ving satisfactory results. In most cases, we suggest permanent hair removal in the upper third, as there will always be some degree of microsomia on the microtic side.
This technique can be considered in our country’s surgical treatment guidelines for Microtia and other auricular deformities.
Funding
The authors declare that they have not received funding.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical considerations
Protection of humans and animals. The authors declare that no experiments involving humans or animals were conducted for this research.
Confidentiality, informed consent, and ethical approval. The authors have followed their institution’s confidentiality protocols, obtained informed consent from patients, and received approval from the Ethics Committee. The SAGER guidelines were followed according to the nature of the study.
Declaration on the use of artificial intelligence. The authors declare that no generative artificial intelligence was used in the writing of this manuscript.